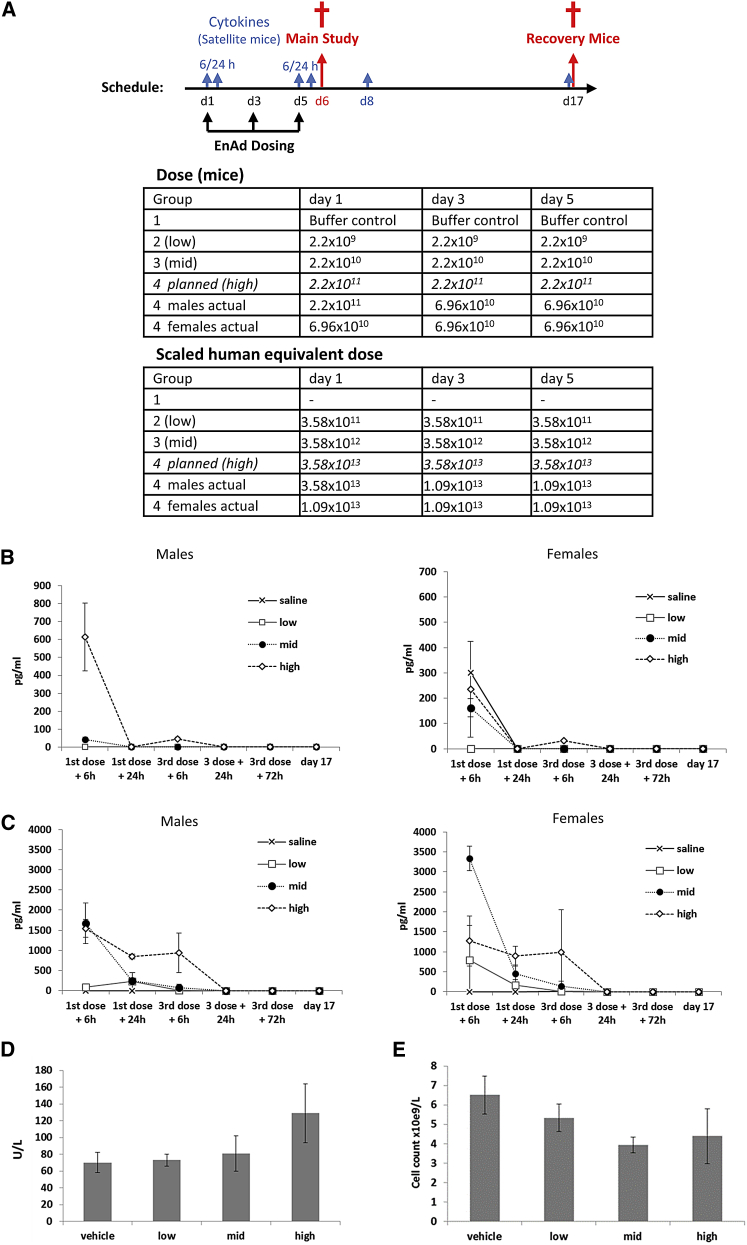
Figure 7.
GLP Acute Toxicology Study in CD-1 Nude Mice
(A) Dosing schedule and groups for acute toxicology study in CD-1 nude mice and the scaled human equivalent doses. Mice were randomized into dosing cohorts each with 25 male and 25 female mice of which eight were evaluated at days 6, 8, and 17. The remaining nine mice were placed in satellite groups and used to assess cytokine levels at each additional time point (n = 3 per time point). The body surface area-based allometric scaling calculations43, 44, 45 were determined according to FDA guidelines. The highest planned dose was not tolerated in two of the male mice and dosing was decreased by approximately half a log for subsequent doses and all female mice. (B and C) IL-6 (B) and MCP-1 (C) levels were determined by flow cytometry using a Cytokine Bead Array Kit. Data represent mean values ± SD (n = 3). (D and E) On day 6, liver alanine transaminase levels (D) and total white blood counts (E) were measured. Data represent mean values ± SD (n = 3).