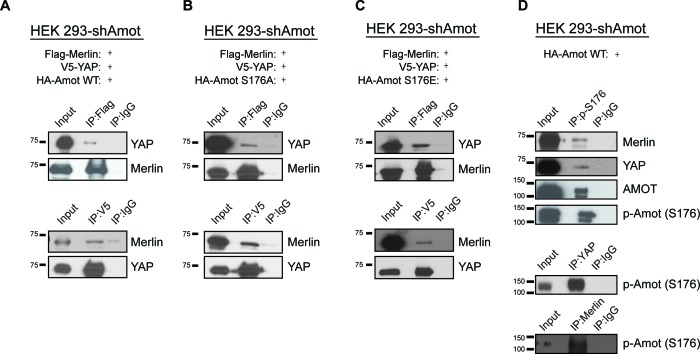
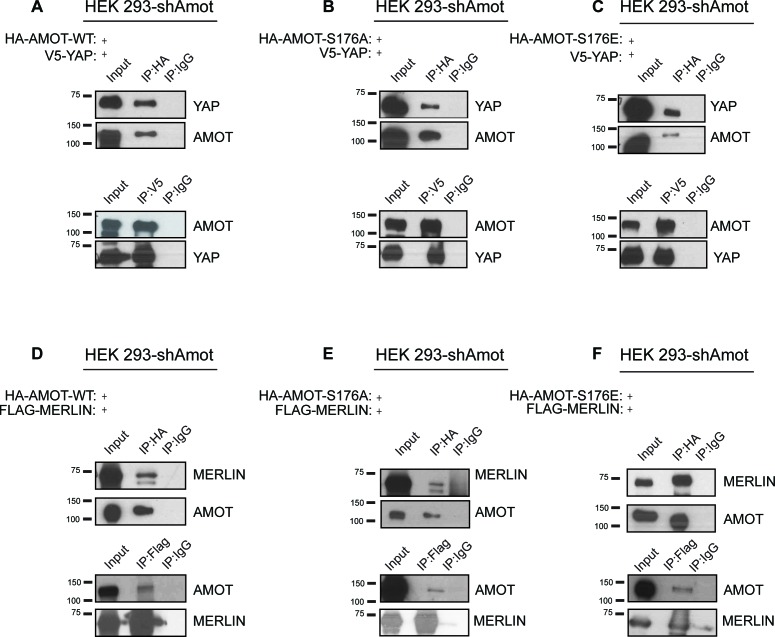
Figure 3. Phosphorylation status of Amot-p130S176 does not impact formation of the Amot/YAP/Merlin complex.
HEK293-shAmot cells were co-transfected with expression plasmids for Flag-Merlin, V5-YAP, and (A) HA-Amot-WT (B) HA-Amot-p130S176A or (C) HA-Amot-p130S176E. Total lysates (input) and Flag or V5 IPs were subjected to immunoblot analysis with anti-YAP and anti-Merlin antibodies as indicated. (D) HEK293-shAmot cells were co-transfected with an expression plasmid for Amot-p130. Total lysates (input) and IPs for phospho-Amot (Ser176), YAP, and Merlin were subjected to immunoblot analysis with indicated antibodies. The blots shown are representative of three independent biological replicates (n = 3).