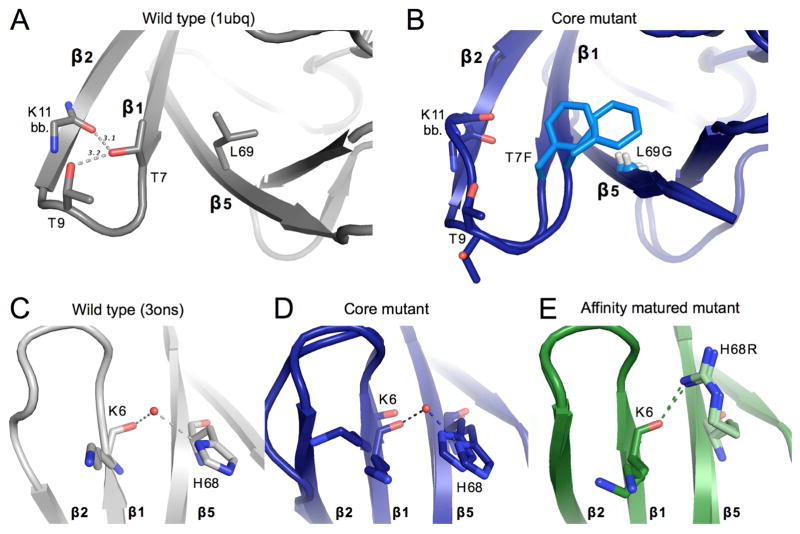
Figure 4. Structural changes upon mutation near β1β2-loop.
A) Key packing and hydrogen bond interactions around mutation sites T7F and L69G. WT ubiquitin (1ubq) is shown in gray. Gray dashed lines show hydrogen bonds existent in WT ubiquitin between residues 7, 9, and 11. Sticks are shown for the side chains of residues 7 and 9, as well as for the backbone of residue 11.
B) Both conformations of the residues shown in panel A are shown for the core mutant. Mutated residues are colored in a lighter blue. The Cα of Gly69 is shown as a small sphere for clarity.
C, D, E) Interactions between residue 6 of β-stand 1 and residue 68 of strand 5 for the wild-type ubiquitin (gray, panel C), of the core mutant (blue, panel D), and the affinity matured mutant (green, panel E). A modeled water appears linking the backbone of residue 6 with the histidine 68 side-chain in both the core mutant and WT (3ons). This interaction is directly replaced in the affinity matured mutant by a hydrogen bond between the new arginine side-chain and the backbone carbonyl of residue 6.
See also Figure S3.