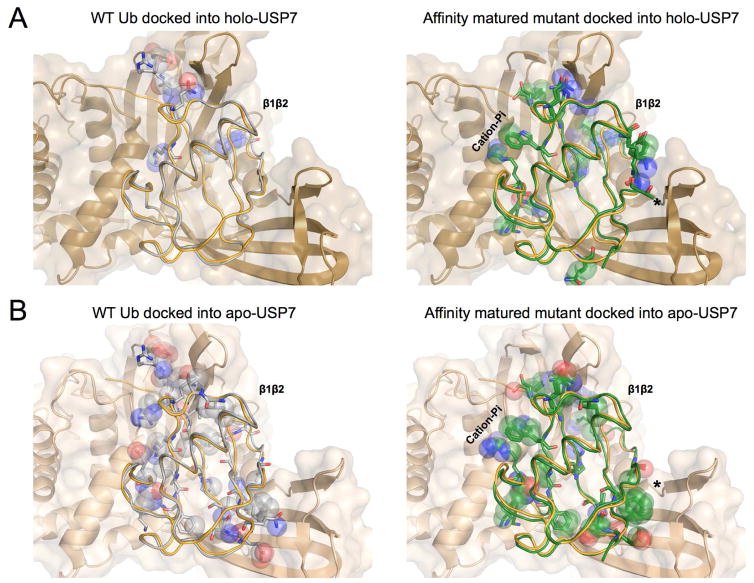
Figure 7. WT and Affinity matured Ubiquitin have distinct patterns of contacts to Apo and holo USP7.
A) Unbound WT Ub (grey - 1ubq) shown in ribbon overlaid with the bound Ub from the holo USP7 structure (orange - 1nbf). Ub-bound USP7 is shown in cartoon and surface (tan - 1nbf). Clashing atoms between the Unbound WT Ub and USP7 are mainly concentrated at the β1β2 loop (top) and shown in spheres with the remainder of the residue shown in sticks.
B) Affinity enhanced mutant (green) shown overlaid with bound Ub from the holo USP7 structure. Although contacts are changed at the β1β2 loop (top), additional clashes, indicative of an altered binding mode or receptor accommodation are spread throughout the protein. An asterisk marks notable changes in clashes between apo and holo-USP7 structures (Panels B & D).
C) Unbound WT Ub (grey -1ubq) shown overlaid with the unbound apo-USP7 structure (lighter brown - 5j7t). Overlay was constructed by alignment of domains to the bound-USP7 (1nbf). Increased clashes throughout the protein show the accommodation of the receptor in the holo form.
D) Affinity matured mutant (green) shown overlaid with the unbound apo-USP7 structure (5j7t). Although clashes are increased in some regions, they are reduced in others, which suggests that the conformational flexibility of the receptor may be exploited by the Affinity matured mutant in the final binding pose. An asterisk marks notable changes in clashes between apo and holo-USP7 structures (Panels B & D).