Abstract
A 27‐year‐old woman with panic disorder taking 20 mg olanzapine daily for 4 months was admitted to Mito Kyodo General Hospital, Mito, Ibaraki, Japan, because of disturbed consciousness with fever, hyperglycemia, hyperosmolarity and elevated creatine phosphokinase. She was diagnosed with a hyperosmolar hyperglycemic state and neuroleptic malignant syndrome. Brain magnetic resonance imaging showed transiently restricted diffusion in the splenium of the corpus callosum, with a high signal intensity on diffusion‐weighted imaging. The neurological abnormalities disappeared along with improvement of metabolic derangements, and the follow‐up magnetic resonance imaging carried out on the 26th day of admission showed complete resolution of the lesions in the splenium of the corpus callosum. These clinical and radiological features are highly suggestive of clinically mild encephalitis/encephalopathy with a reversible splenial lesion. The first case of mild encephalitis/encephalopathy with a reversible splenial lesion caused by olanzapine‐induced hyperosmolar hyperglycemic state and neuroleptic malignant syndrome is reported.
Keywords: Diabetes mellitus, Neuroleptic malignant syndrome, Olanzapine
Introduction
Clinically mild encephalitis/encephalopathy with a reversible splenial lesion (MERS) is a new clinical‐radiological syndrome. MERS is characterized by reversible magnetic resonance imaging (MRI) lesions with transiently restricted diffusion in the splenium of the corpus callosum (SCC)1. Various etiologies, such as infection and metabolic abnormalities, are involved in the development of MERS2.
Olanzapine, an atypical antipsychotic, is widely used for schizophrenia or bipolar disorder. However, concerns have been expressed about metabolic adverse effects with the use of olanzapine, such as diabetes mellitus including diabetic ketoacidosis and hyperosmolar hyperglycemic state (HHS) or neuroleptic malignant syndrome (NMS)3.
The first case in which a patient developed MERS caused by olanzapine‐induced HHS and NMS is described.
Case Report
The patient was a 27‐year‐old woman with panic disorder taking 20 mg olanzapine daily for 4 months. She was diagnosed with panic disorder at 25 years‐of‐age, when her random blood glucose (94 mg/dL) and glycated hemoglobin (4.9%) were normal. After starting paroxetine, her weight increased by 20 kg over 1.5 years as a result of hyperphagia and excessive drinking of soft drinks. Paroxetine was switched to olanzapine 4 months earlier. She was referred to Mito Kyodo General Hospital, Mito, Ibaraki, Japan, due to a 3‐day history of excessive thirst and disturbed consciousness. She had impaired consciousness (Glasgow Coma Scale E3V3M5). Her blood pressure, pulse rate, and temperature were 154/84 mmHg, 125/min and 37.6°C, respectively, on admission. Laboratory data showed HHS with random blood glucose of 762 mg/dL, sodium of 182 mEq/L and serum osmolality of 430 mOsm/L, as shown in Table 1. Urine dipstick analysis showed ketones, proteins and glucose. A lumbar puncture showed clear cerebrospinal fluid with no evidence of infection (Table 1). No bacteria were detected in blood, urine and cerebrospinal fluid cultures. Rapid antigen‐detection assays from a nasopharyngeal swab were negative for influenza A and B. There were no increases of serum immunoglobulin M virus antibodies (Table 1), and serum antinuclear antibody was negative. The patient was diagnosed with NMS, because her plasma creatine phosphokinase, white blood cell count and temperature were elevated to 8,530 IU/L, 11,400/μL and 39.5°C, respectively, and hypertension, tachycardia and altered consciousness developed. Furthermore, T2‐weighted, diffusion‐weighted and fluid‐attenuated inversion recovery images of brain MRI carried out on the second hospital day showed high‐intensity lesions in the SCC (Figure 1a), with decreased apparent diffusion coefficient values (Figure 1b), suggesting MERS.
Table 1.
Laboratory data on admission
| Urinalysis | ALT | 68 IU/L | |
| pH | 5 | LDH | 352 IU/L |
| Protein | (+/−) | CK | 806 IU/L |
| Glucose | >2,000 mg/dL | T‐bil | 1 mg/dL |
| Blood | (1+) | CRP | 4.12 mg/dL |
| Ketone | (1+) | PG | 762 mg/dL |
| CBC | HbA1c | 13.4% | |
| WBC | 8,300/μL | GA | 37.4% |
| RBC | 582 × 104/μL | Serum ketone | 1,723 μmol/L |
| Hb | 18 g/dL | Serum osmolality | 430 mOsm/L |
| Ht | 35.2% | TSH | 2.89 μIU/mL |
| Plt | 54.3 × 104/μL | F‐T4 | 1 ng/dL |
| Biochemistry | BGA | ||
| TP | 7.6 g/dL | pH | 7.385 |
| Alb | 3.9 g/dL | pO2 | 93.4 mmHg |
| BUN | 50 mg/dL | pCO2 | 37.6 mmHg |
| Cre | 1.5 mg/dL | HCO3− | 22 mmol/L |
| Na | 182 mEq/L | BE | −2.5 mmol/L |
| K | 4.1 mEq/L | CSF analysis | |
| Cl | 131 mEq/L | Leukocytes | 2/μL |
| ALP | 436 IU/L | Protein | 44 mg/dL |
| γ‐GT | 30 IU/L | Glucose | 149 mg/dL |
| AST | 48 IU/L | Autoantibody | |
| Viral antibodies | ANA | <40 | |
| HSV IgM (EIA) | 0.4 (−) | ||
| HHV‐6 IgM (FA) | <10 (−) | ||
| EBV IgM (FA) | <10 (−) | ||
| CMV IgM (EIA) | 0.49 (−) | ||
γ‐GT, γ‐glutamyl transpeptidase; Alb, albumin; ALP, alkaline phosphatase; ANA, anti‐nuclear antibody; AST, aspartate aminotransferase; BGA, blood gas analysis; BE, base excess; BUN, blood urea nitrogen; CBC, complete blood count; CK, creatine kinase; CMV, cytomegalovirus; Cre, creatine; CRP, C‐reactive protein; CSF, cerebrospinal fluid; EBV, Epstein–Barr virus; EIA, enzyme immunoassay; FA, fluorescent‐antibody; GA, glycoalbumin; Hb, hemoglobin; HbA1c, glycated hemoglobin; HHV‐6, human herpesvirus 6; HSV, herpes simplex virus; Ht, hematocrit; IgM, immunoglobulin M; LDH, lactate dehydrogenase; PG, plasma glucose; Plt, platelets; RBC, red blood cells; T‐bil, total bilirubin; TP, total protein; TSH, thyroid‐stimulating hormone; WBC, white blood cells.
Figure 1.
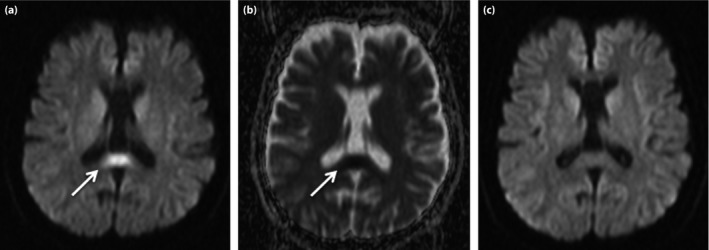
Splenium of the corpus callosum lesions on brain magnetic resonance imaging. (a) Diffusion‐weighted imaging and (b) the apparent diffusion coefficient map on the second hospital day, and (c) diffusion‐weighted imaging on the 26th day of admission.
The patient was treated with rehydration, continuous intravenous insulin infusion and subsequently with ventilator support for 5 days because of her impaired consciousness. Olanzapine was promptly withdrawn. Her body temperature and both the plasma creatine phosphokinase and sodium levels were normalized by the 14th day of admission. After continuous insulin infusion, blood glucose was controlled by multiple daily insulin injections, followed by oral therapy with vildagliptine. Serum C‐peptide reactivity measured during multiple daily insulin injections was normal at 2.37 ng/mL (reference range 0.78–5.19 ng/mL). Her consciousness improved completely, and the abnormal SCC lesions were resolved on a follow‐up MRI carried out on the 26th day of admission (Figure 1c).
Discussion
A patient with both HHS and NMS that developed 4 months after starting olanzapine treatment was presented. Furthermore, these metabolic abnormalities induced MERS. To the best of our knowledge, there have not been any reported cases of MERS concomitant with HHS and NMS caused by olanzapine.
In recent years, a reversible lesion with transiently reduced diffusion in the SCC has been reported in patients with clinically mild encephalitis/encephalopathy, which is referred to as MERS. The SCC lesions on MRI show a hypointense signal on T1, and hyperintense signals on T2 and fluid‐attenuated inversion recovery sequences. Restricted diffusion is also observed on diffusion‐weighted imaging, with low apparent diffusion coefficient values within the lesions1. The SCC lesions disappeared or improved significantly on follow‐up MRI carried out a couple of months later. The common neurological symptoms are delirious behavior, followed by consciousness disturbance and seizures, all of which resolve completely within a month. In the present case, consciousness disturbance without evidence of cerebrospinal infection resolved within 2 weeks after admission. Taken together with typical SCC lesions on MRI, the diagnosis of this patient was MERS.
MERS has been shown to be associated with several disorders, including infection, collagen disease, seizures and anti‐epileptic drug withdrawal, high‐altitude cerebral edema, and metabolic disturbances2, 4, 5, 6. Although hypoglycemia was associated with MERS7, a relationship between hyperglycemia and MERS has not yet been suggested. Takanashi et al.6 showed that the plasma sodium level was much lower in MERS than in other encephalopathies. In contrast, Al‐Edrus et al.4 reported a case of bipolar disorder on multiple neuropsychiatric drugs presenting with hypernatremia and NMS, which led to the development of MERS. We therefore suggest that hypernatremia induced by HHS and NMS caused the MERS in the present case. Considering the diversity of etiologies in MERS, HHS or NMS might also directly contribute to the pathophysiology of MERS. In the present case, there was no evidence of infectious or collagen diseases known to cause MERS.
Plasma glucose and glycated hemoglobin were normal in the present patient before starting paroxetine. Although plasma glucose was not measured thereafter, the weight gain and change of eating behavior induced by paroxetine might have led to the metabolic disturbance. Olanzapine, which replaced paroxetine 4 months earlier, finally induced HHS and NMS. Because the serum C‐peptide reactivity remained normal, it was unlikely that the patient suffered from type 1 diabetes mellitus. Although the precise mechanism of olanzapine‐induced hyperglycemia is as yet unclear, hyperphagia, weight gain, insulin resistance or impairment of β‐cell function would be involved in the development of diabetes8. Cases of olanzapine‐induced hyperglycemia have had variable onset from 10 days to years, and have not been dose‐dependent9. This risk was particularly robust in the first 3 months of olanzapine use.
NMS is a relatively rare, but potentially fatal, side‐effect of antipsychotic medications including olanzapine. According to the diagnostic criteria of NMS by Levenson et al.10, major manifestations consist of fever, rigidity and elevated plasma creatine phosphokinase, whereas minor manifestations include tachycardia, abnormal blood pressure, tachypnea, altered consciousness, diaphoresis and leukocytosis. Because two of the three major manifestations, excluding rigidity, and five of the six minor manifestations, excluding tachypnea, were observed, the diagnosis of NMS was made in the present case.
In summary, the first case in which a patient taking olanzapine developed MERS was described. Therefore, MERS should be considered in the differential diagnosis of consciousness disturbance in a patient taking olanzapine who develops HHS and/or NMS.
Disclosure
The authors declare no conflict of interest.
J Diabetes Investig 2017; 8: 392–394
References
- 1. Takanashi J. Two newly proposed infectious encephalitis/encephalopathy syndromes. Brain Dev 2009; 31: 521–528. [DOI] [PubMed] [Google Scholar]
- 2. Garcia‐Monco JC, Cortina IE, Ferreira E, et al Reversible splenial lesion syndrome (RESLES): what's in a name? J Neuroimaging 2011; 21: e1–e14. [DOI] [PubMed] [Google Scholar]
- 3. Ahuja N, Palanichamy N, Mackin P, et al Olanzapine‐induced hyperglycaemic coma and neuroleptic malignant syndrome: case report and review of literature. J Psychopharmacol 2010; 24: 125–130. [DOI] [PubMed] [Google Scholar]
- 4. Al‐Edrus S, Norzaini R, Chua R, et al Reversible splenial lesion syndrome in neuroleptic malignant syndrome. Biomed Imaging Interv J 2009; 5: e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maeda M, Tsukahara H, Terada H, et al Reversible splenial lesion with restricted diffusion in a wide spectrum of diseases and conditions. J Neuroradiol 2006; 33: 229–236. [DOI] [PubMed] [Google Scholar]
- 6. Takanashi J, Tada H, Maeda M, et al Encephalopathy with a reversible splenial lesion is associated with hyponatremia. Brain Dev 2009; 31: 217–220. [DOI] [PubMed] [Google Scholar]
- 7. Kim JH, Choi JY, Koh SB, et al Reversible splenial abnormality in hypoglycemic encephalopathy. Neuroradiology 2007; 49: 217–222. [DOI] [PubMed] [Google Scholar]
- 8. Wirshing DA, Spellberg BJ, Erhart SM, et al Novel antipsychotics and new onset diabetes. Biol Psychiatry 1998; 44: 778–783. [DOI] [PubMed] [Google Scholar]
- 9. Sneed KB, Gonzalez EC. Type 2 diabetes mellitus induced by an atypical antipsychotic medication. J Am Board Fam Pract 2003; 16: 251–254. [DOI] [PubMed] [Google Scholar]
- 10. Levenson JL. Neuroleptic malignant syndrome. Am J Psychiatry 1985; 142: 1137–1145. [DOI] [PubMed] [Google Scholar]


