Abstract
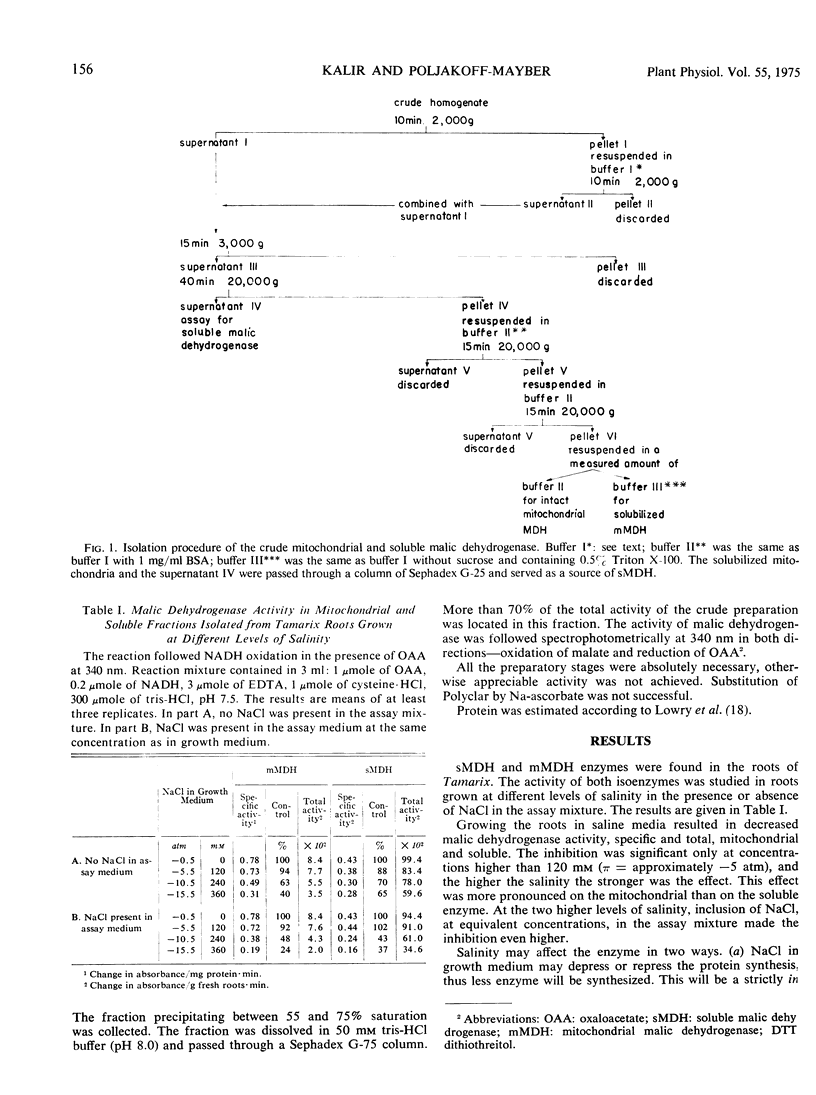
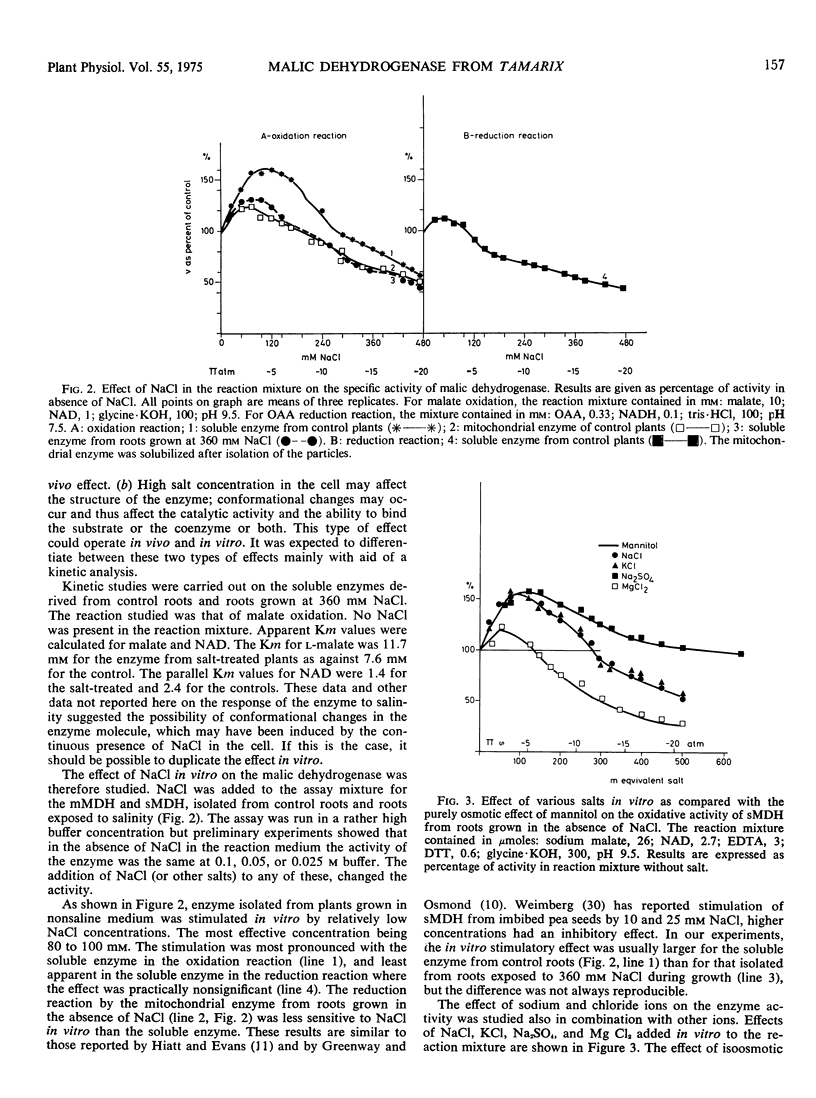
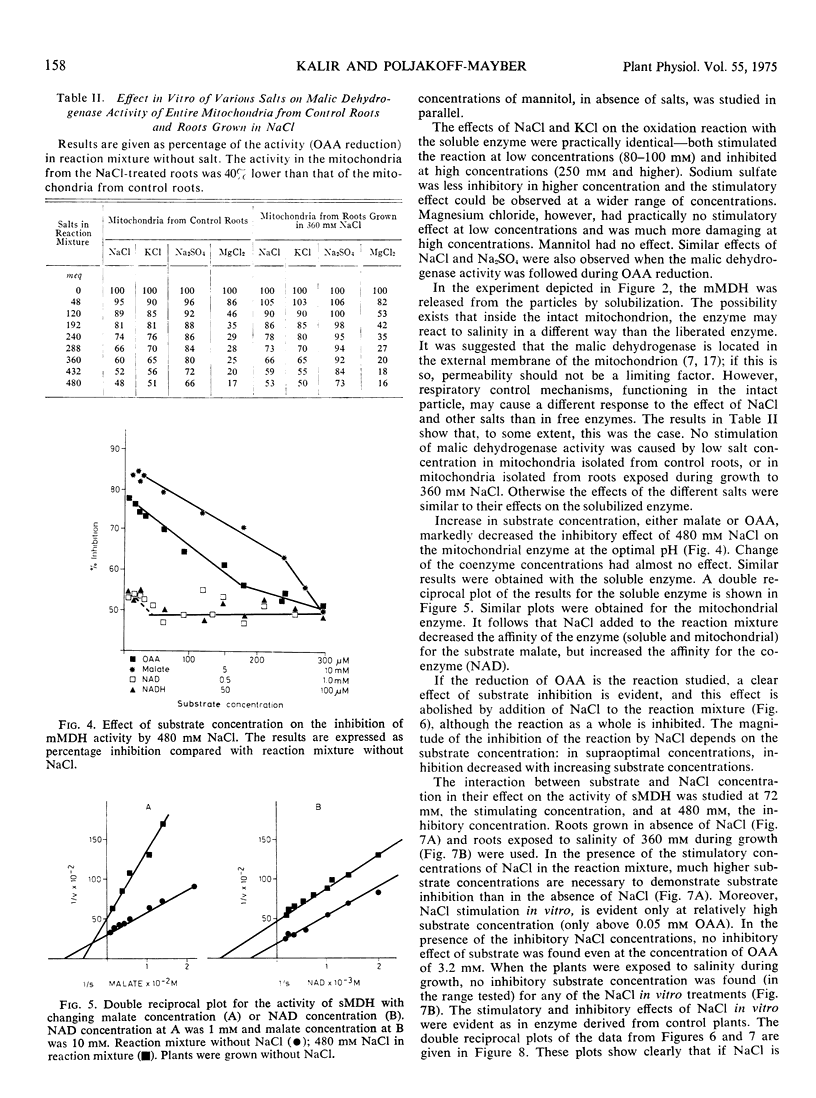
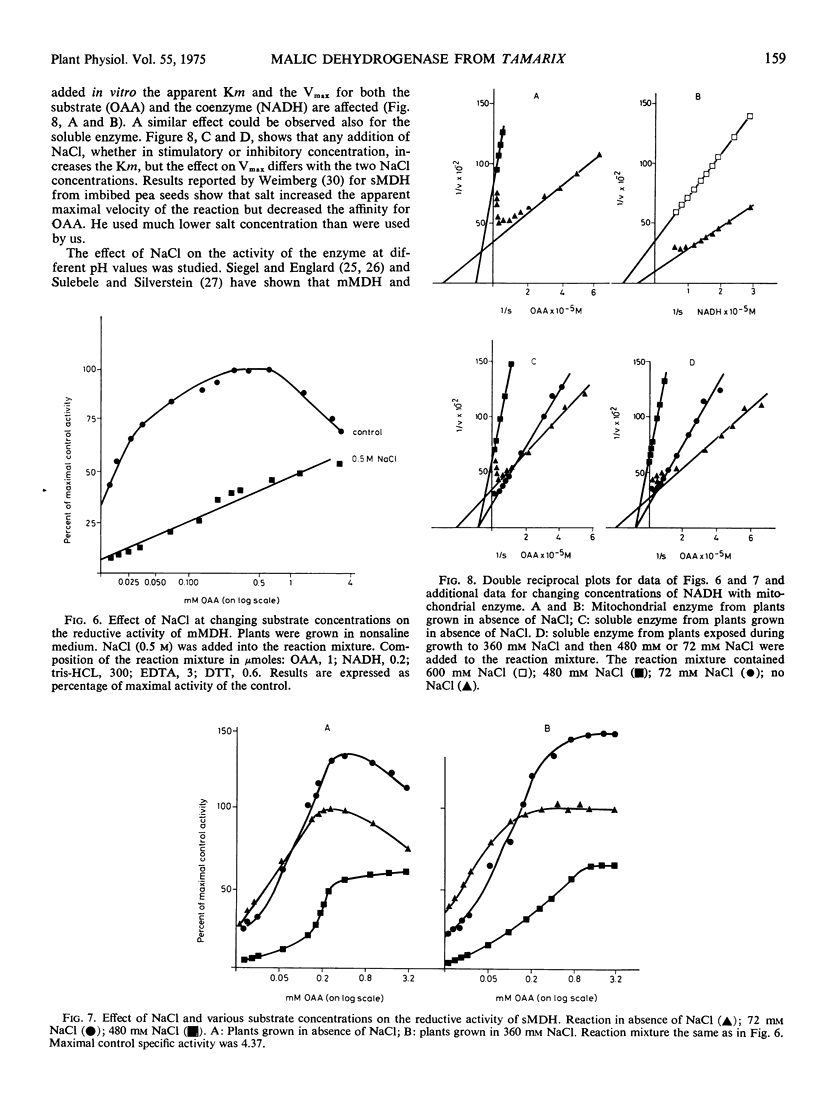
Soluble and mitochondrial malic dehydrogenases (MDH) were isolated from root tips of the halophyte Tamarix tetragyna L. grown in the presence and absence of NaCl. The activity of the enzymes isolated from root tips grown in the presence of NaCl was lower than that of the enzymes isolated from roots grown in absence of NaCl. The mitochondrial MDH was much more sensitive to salinity than the soluble MDH. The soluble enzyme from roots grown in NaCl had a higher Km for malate and lower Km for NAD than enzyme from the control roots. Addition of NaCl in vitro at 72 mM significantly stimulated the reductive activity of soluble MDH, while higher NaCl concentrations (240 mM and above) depressed enzyme activity. The inhibition of enzyme activity by various salts was found to be in the order MgCl2 > NaCl = KCl > Na2SO4. Mannitol at equiosmotic concentrations had no effect. Substrate inhibition, typical for oxaloacetate oxidation, was not observed at high NaCl concentrations in vitro and high substrate concentrations neutralized the inhibitory effect of NaCl. Increased coenzyme concentrations had no effect. In vitro NaCl increased the Km for malate and oxaloacetate already at relatively low concentrations. At the same time NaCl decreased the Km for NAD and NADH. The inhibitory effect of NaCl on enzyme activity seems not to be due to the effect on the Km alone. Soluble and mitochondrial MDH had different responses to pH changes, mitochondrial MDH being more sensitive. Mitochondrial MDH released from the particles had a similar response to that of the entire particles. Changes of pH modified the effect of NaCl on enzyme activity. It was postulated that NaCl apparently induces conformational changes in the enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken D. M., Wicken A. J., Brown A. D. Properties of a halophil nicotinamide--adenine dinucleotide phosphate-specific isocitrate dehydrogenase. Preliminary studies of the salt relations and kinetics of the crude enzyme. Biochem J. 1970 Jan;116(1):125–134. doi: 10.1042/bj1160125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAXTER R. M. An interpretation of the effects of salts on the lactic dehydrogenase of Halobacterium salinarium. Can J Microbiol. 1959 Feb;5(1):47–57. doi: 10.1139/m59-006. [DOI] [PubMed] [Google Scholar]
- DAVIES D. D., KUN E. Isolation and properties of malic dehydrogenase from ox-heart mitochondria. Biochem J. 1957 Jun;66(2):307–316. doi: 10.1042/bj0660307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta P. Homoserine dehydrogenase of Rhodospirillum rubrum. Conformational changes in the presence of substrates and modifiers. Biochemistry. 1971 Feb 2;10(3):402–408. doi: 10.1021/bi00779a007. [DOI] [PubMed] [Google Scholar]
- Greenway H. Salt responses of enzymes from species differing in salt tolerance. Plant Physiol. 1972 Feb;49(2):256–259. doi: 10.1104/pp.49.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMES P. K., HALVORSON H. O. PURIFICATION OF A SALT-REQUIRING ENZYME FROM AN OBLIGATELY HALOPHILIC BACTERIUM. J Bacteriol. 1965 Aug;90:312–315. doi: 10.1128/jb.90.2.312-315.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. S., Miller A. B. Nature of the inactivation of the isocitrate dehydrogenase from an obligate halophile. J Bacteriol. 1970 Jun;102(3):677–681. doi: 10.1128/jb.102.3.677-681.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. S., Miller A. B. Purification and reversible inactivation of the isocitrate dehydrogenase from an obligate halophile. J Bacteriol. 1969 Jul;99(1):161–168. doi: 10.1128/jb.99.1.161-168.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lieberman M. M., Lanyi J. K. Threonine deaminase from extremely halophilic bacteria. Cooperative substrate kinetics and salt dependence. Biochemistry. 1972 Jan 18;11(2):211–216. doi: 10.1021/bi00752a011. [DOI] [PubMed] [Google Scholar]
- RAVAL D. N., WOLFE R. G. Malic dehydrogenase. IV. pH dependence of the kinetic parametrs. Biochemistry. 1962 Nov;1:1118–1123. doi: 10.1021/bi00912a024. [DOI] [PubMed] [Google Scholar]
- Rafaeli-Eshkol D., Avi-Dor Y. Studies on halotolerance in a moderately halophilic bacterium. Effect of betaine on salt resistance of the respiratory system. Biochem J. 1968 Oct;109(4):687–691. doi: 10.1042/bj1090687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEGEL L., ENGLARD S. Beef-heart malic dehydrogenases. I. Properties of the enzyme purified from extracts of acetone-dried powders. Biochim Biophys Acta. 1961 Nov 25;54:67–76. doi: 10.1016/0006-3002(61)90938-6. [DOI] [PubMed] [Google Scholar]
- SIEGEL L., ENGLARD S. Beef-heart malic dehydrogenases. III. Comparative studies of some properties of M-malic dehydrogenase and S-malic dehydrogenase. Biochim Biophys Acta. 1962 Oct 8;64:101–110. doi: 10.1016/0006-3002(62)90763-1. [DOI] [PubMed] [Google Scholar]
- Sulebele G., Silverstein E. Malate dehydrogenase and aspartate aminotransferase of Phycomyces blakesleeanus. Arch Biochem Biophys. 1969 Sep;133(2):425–435. doi: 10.1016/0003-9861(69)90472-x. [DOI] [PubMed] [Google Scholar]
- Ting I. P. Malic dehydrogenases in corn root tips. Arch Biochem Biophys. 1968 Jul;126(1):1–7. doi: 10.1016/0003-9861(68)90552-3. [DOI] [PubMed] [Google Scholar]


