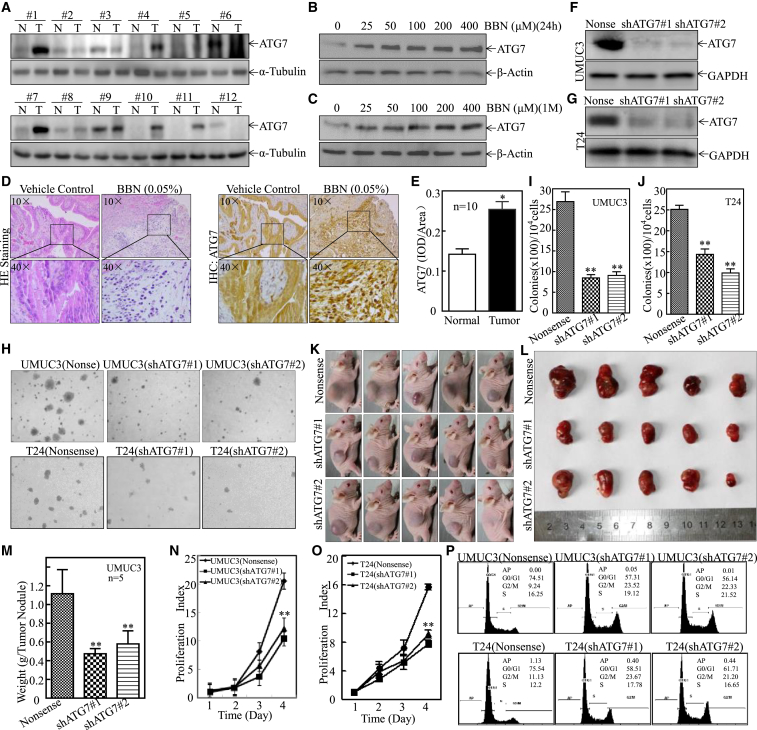
Figure 1.
ATG7 Was Overexpressed in Human BCs, BBN-Induced Human Normal Bladder Urothelial UROtsa Cells, and BBN-Induced Highly Invasive Mouse BCs and Was Crucial for Anchorage-Independent Growth In Vitro and Tumorigenicity of Human BC Cells In Vivo
(A) Total protein lysates were prepared from human bladder cancerous tissues (T) and paired adjacent normal tissues (N) among 12 patients diagnosed with invasive BC and subjected to western blot analysis for determining the ATG7 protein expression profile. (B and C) Human normal bladder urothelial cell line UROtsa cells were treated with BBN at different doses for 24 hr (B) or for 1 month (C). The total cell lysates were subjected to western blot to determine the expression of ATG7. β-Actin was used as a protein loading control. (D) H&E staining and IHC staining were performed to evaluate morphology and ATG7 expression in BBN-induced mouse invasive BCs. The IHC images were captured using the AxioVision Rel.4.6 computerized image system. (E) The ATG7 protein expression levels were analyzed by calculating the integrated IOD/area using Image-Pro Plus version 6.0. Three independent experiments were performed, and Student’s t test was utilized to determine the p values. An asterisk indicates a significant increase from vehicle-treated mice (*p < 0.05). (F and G) ATG7 knockdown constructs were stably transfected into UMUC3 (F) and T24 (G) cells, respectively. The knockdown efficiency of ATG7 protein was assessed by western blotting. (H) UMUC3(shATG7#1) cells, UMUC3(shATG7#2) cells versus UMUC3(Nonsense) cells or T24(shATG7#1) cells, and T24 (shATG7#2) cells versus T24 (Nonsense) cells were subjected to an anchorage-independent soft agar assay using the protocol described in Materials and Methods. Representative images of colonies of the indicated cells were taken under an Olympus DP71. (I and J) The number of colonies was counted with more than 32 cells of each colony, and the results are presented as colonies per 104 cells. The bars show mean ± SD from three independent experiments. Double asterisks indicate a significant decrease in comparison with nonsense control transfectants (**p < 0.05). (K–M) Athymic nude mice were subcutaneously injected with UMUC3(Nonsense), UMUC3(shATG7#1), and UMUC3(shATG7#2) transfectants (2 × 106 suspended in 100 μL PBS) into the right axillary region as described in Materials and Methods. Four weeks after cell injection, the mice were sacrificed, and the tumors were surgically removed and photographed. (K and L) as well as weighed (M). Bars represent mean ± SD from each group of five mice. Student’s t test was utilized to determine the p value. Double asterisks indicate a significant decrease in comparison with UMUC3(Nonsense) transfectants (**p < 0.05). (N and O) UMUC3 transfectants and T24 transfectants, as indicated, were plated in 96-well plates at a density of 5,000 cells/well. The cell culture medium was then replaced with 0.1% FBS DMEM or 0.1% FBS DMEM-F12 (1:1) and cultured for 12 hr. The medium was replaced with normal medium and cultured for another 1, 2, 3, or 4 days. Subsequently, an ATP activity assay was performed using the protocol described in Materials and Methods. Double asterisks indicate a significant decrease from the nonsense control. (P) The indicated cells (2 × 105) were seeded into a 6-well plate and cultured overnight. Following synchronization in 0.1% FBS for 12 hr, the medium was replaced with 10% FBS DMEM or 5% FBS DMEM-F12 (1:1) for another 12 hr, and then the cells were stained with propidium iodide for cell cycle analysis as described in Materials and Methods. The results represent one of three independent experiments.