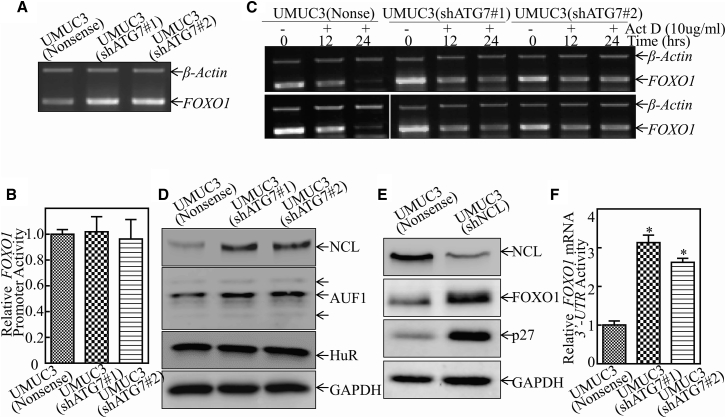
Figure 5.
ATG7 Overexpression Decreased FOXO1 mRNA Stabilization by Regulating Its mRNA 3′ UTR Activity
(A) UMUC3(shATG7#1), UMUC3(shATG7#2), and UMUC3(Nonsense) cells were cultured in 6-well plates until the cell density reached 70%–80%. Following synchronization for 12 hr, the medium was then replaced with 10% FBS DMEM for another 12 hr. Then the cells were extracted for total RNA with TRIzol reagent. RT-PCR was used to determine FOXO1 mRNA expression, and β-actin was used as an internal control. (B) The human FOXO1 promoter-driven luciferase reporter was used to evaluate its promoter transcription activity in the indicated transfectants. The results were normalized by internal TK activity. (C) UMUC3(shATG7#1), UMUC3(shATG7#2), and UMUC3(Nonsense) cells were seeded into 6-well plates. After synchronization, the indicated cells were treated with Act D for the indicated times. Total RNA was then isolated and subjected to RT-PCR analysis for mRNA levels of FOXO1, and β-actin was used as an internal control. (D) The indicated cell extracts were subjected to western blot for determination of NCL, AUF1, and HuR protein expression. GAPDH was used as a protein loading control. (E) NCL knockdown constructs were stably transfected into UMUC3 cells. The knockdown efficiency of NCL protein and the expression of FOXO1 and p27 were evaluated by western blotting. GAPDH was used as a protein loading control. (F) The pMIR-FOXO1 3′ UTR mRNA reporter was transiently transfected into the indicated cells, and the luciferase activity of each transfectant was evaluated. The luciferase activity is presented as relative to nonsense transfectant, normalized by using pRL-TK as an internal control. The bars show mean ± SD from three independent experiments. The asterisk indicates a significant increase in UMUC3(shATG7) in comparison with nonsense transfectant (*p < 0.05).