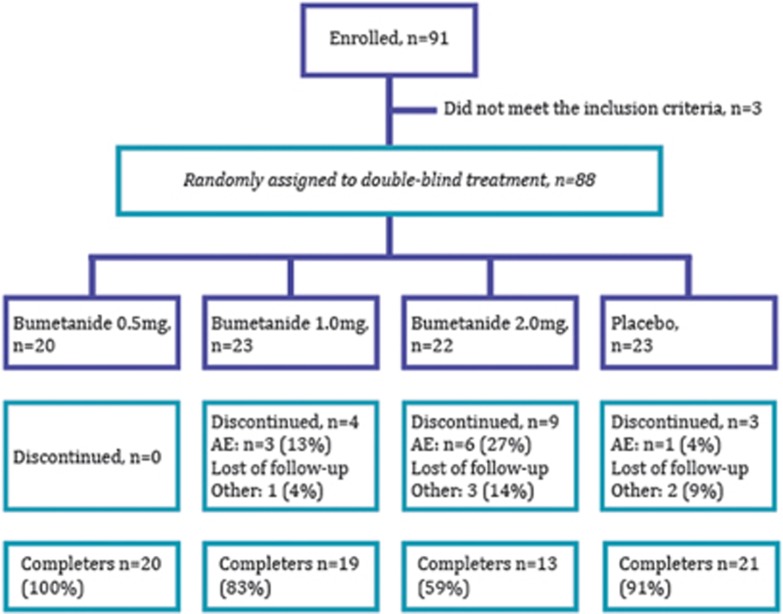
Figure 1.
Patient flow in each treatment groups throughout the study. The enrolled and the included patients are noted followed by the four groups who received 0.5, 1.0 and 2 mg twice daily or placebo. Then the number of patients discontinuing the trial because of an adverse event (AE) or loss to follow-up is indicated, followed by the number of patients who completed the trial for each subgroup. One of the three discontinued patients in the placebo group withdrew after the visit on day 90 and was considered as a completer.