Abstract
Calcium channels control the inflow of calcium ions into cells and are involved in diverse cellular functions. The CACNA1C gene polymorphism rs1006737 A allele has been strongly associated with increased risk for bipolar disorder (BD) and with modulation of brain morphology. The medial prefrontal cortex (mPFC) has been widely associated with mood regulation in BD, but the role of this CACNA1C polymorphism in mPFC morphology and brain aging has yet to be elucidated. One hundred seventeen euthymic BD type I subjects were genotyped for CACNA1C rs1006737 and underwent 3 T three-dimensional structural magnetic resonance imaging scans to determine cortical thickness of mPFC components (superior frontal cortex (sFC), medial orbitofrontal cortex (mOFC), caudal anterior cingulate cortex (cACC) and rostral anterior cingulate cortex (rACC)). Carriers of the CACNA1C allele A exhibited greater left mOFC thickness compared to non-carriers. Moreover, CACNA1C A carriers showed age-related cortical thinning of the left cACC, whereas among A non-carriers there was not an effect of age on left cACC cortical thinning. In the sFC, mOFC and rACC (left or right), a negative correlation was observed between age and cortical thickness, regardless of CACNA1C rs1006737 A status. Further studies investigating the direct link between cortical thickness, calcium channel function, apoptosis mechanism and their underlying relationship with aging-associated cognitive decline in BD are warranted.
Introduction
The medial prefrontal cortex (mPFC) is part of a brain circuit involved in emotional expression and mood modulation.1, 2, 3, 4, 5 This limbic area displays extensive abnormalities in bipolar disorder (BD).6, 7, 8, 9, 10 A single-nucleotide polymorphism on the CACNA1C gene, which encodes the calcium v1.2 L-type voltage-gated channel, has been strongly associated with BD.11, 12, 13 Moreover, this single-nucleotide polymorphism has been associated with cognitive performance14, 15 and regional gray matter (GM) volume changes.1, 16, 17 However, there are no studies investigating the influence of the CACNA1C risk allele on mPFC morphology, including cortical thickness or brain aging in BD.
The orbitalfrontal (mOFC) part of the mPFC has widespread connections to the limbic area and adjacent PFC regions.18 This same limbic region has also been linked to mood modulation in BD;19 for instance, enhanced connectivity between the mPFC and amygdala has been reported in BD patients viewing sad stimuli.20 The presence of high connectivity between mPFC and insula, a key region for emotional processing, has also been reported in BD.21 Current evidence supports a model of abnormal anatomic connections between PFC and limbic brain structures.22
The CACNA1C gene encodes to the α1-C subunit of the L-type voltage-gated calcium channel. The influx of Ca2+ into neurons activates pathways that rely on Ca2+, including neurotransmitter release, calmodulin-dependent protein kinase II and protein kinase C.23 The activation of calcineurin inhibits the activity of ionotropic glutamate receptors via receptor desphosphorylation following the Ca2+-dependent activation of calmodulin.24 Moreover, the activation of calcium v1.2 L-type voltage-gated channel permits cellular influx of calcium following temporary changes in the membrane potential, which activates downstream pathways of genetic transcription, very important to brain plasticity, such as for brain-derived neurotrophic factor.25, 26 This way, CACNA1C influx of Ca2+ into neurons has neurotrophic properties and works with glutamate ionotropic channels to regulate intracellular Ca2+. The CACNA1C risk variant for BD consists of the presence of the A allele of the single-nucleotide polymorphism rs1006737. This single-nucleotide polymorphism is considered a functional polymorphism because its A/A genotype has been associated with greater CACNA1C messenger RNA expression in the PFC compared with G/G or A/G genotypes.27 Moreover, it has been reported that BD carriers of the A allele have increased amygdala activity during emotional processing tasks compared to non-carriers. A recent study reported that BD subjects carrying the CACNA1C risk allele A had higher levels of intracellular calcium compared to healthy controls (HCs).28
Cortical GM volume is an unspecific measure of brain morphology because it is the product of both cortical thickness and area. Therefore, a decrease in cortical volume may suggest reduced thickness and/or area. The two constituent components of GM result from well-differentiated ontogenic stages during corticogenesis29 and appear to have independent genetic etiology.30 Cortical neurons are arranged into ontogenetic columns that are located perpendicular to the cortical surface. Studies investigating the impact of CACNA1C on regional brain morphology are less extensive in BD patients than in HCs. In controls, there have been reports of the A allele increasing GM,16, 31 brainstem,17 right amygdala and right hypothalamus1 volumes, whereas reports in BD patients are sparse and have focused on different brain structures.
To the best of our knowledge, the only study investigating the impact of CACNA1C on cortical thickness32 in BD type I patients showed negative results for a sample of 71 BD type I patients. Tesli et al.32 reported no effect of CACNA1C rs1006737 on frontal, parietal, temporal or total cortical thickness.
Thus, the intention of the present study was to investigate whether the presence of CACNA1C risk allele A influences cortical thickness in the main components of the mPFC (mPFC (superior frontal (sFC), mOFC, caudal anterior (cACC) and rostral anterior (rACC) cingulate)).
Materials and methods
One hundred seventeen (78 females, 18–45 years) BD type I subjects during euthymia were included in this study. Diagnoses were reached by skilled psychiatrists using the Structured Clinical Interview (SCID-I/P)33 for DSM-IV TR.34 All BD patients had been euthymic for at least 2 months prior to the scanning session. Patients were allowed to be using a combination of antidepressants, lithium, anticonvulsants and antipsychotics according to the clinical criteria without any change on dosage or substance for at least 2 months prior to inclusion in this study. Individuals with neurological disorders or medical disorders, head trauma or current/past (3 months) substance abuse, as well as those who had been treated with electroconvulsive therapy in the last 6 months, were excluded. The Young Mania Rating Scale35 and the Hamilton Depression Rating Scale-21 (ref. 36) were used to assess residual subthreshold depressive and manic symptoms. Euthymia was defined as <7 Young Mania Rating Scale and <7 Hamilton Depression Rating Scale-21. The patients also fulfilled the DSM-IV criteria for remission.
The research ethics committee of the Hospital de Clinicas, University of Sao Paulo (CAPPesq), approved the study. Written informed consent was obtained from all study participants.
Image acquisition
Magnetic resonance imaging was executed using an Intera Achieva 3.0-T system (Philips, Best, The Netherlands) and an eight-channel head coil. Sagittal three-dimensional T1-weighted anatomical images with isotropic 1mm3 resolution were obtained with a fast-field echo sequence (TR=7 ms; echo time (TE)=3.2 ms; inversion time (TI)=900 ms; flip angle=8º). Three-dimensional T1-weighted magnetic resonance images were analyzed with the program FreeSurfer v.5.1.0 (The General Hospital, Boston, MA, USA) to obtain volumes for structures, automatically and non-interactively, in right and left hemispheres. Intracranial volume was also measured with the same software for normalization purposes.
Cortical reconstruction and volumetric segmentation were performed with the Freesurfer image analysis suite, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). The technical details of these procedures are described elsewhere.37, 38, 39, 40, 41, 42, 43, 44, 45, 46 Briefly, this processing includes removal of non-brain tissue using a hybrid watershed/surface deformation technique,47 automated Talairach transformation, segmentation of subcortical white matter and deep GM volumetric structures (including hippocampus, amygdala, caudate, putamen and ventricles),41, 44 intensity normalization,48 tessellation of the GM white matter boundary, automated topology correction,40, 49 and surface deformation following intensity gradients to optimally place the gray/white matter and GM/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class.37, 38, 39 Once the cortical models are complete, a number of deformable procedures can be performed for further data processing and analysis including surface inflation,43 registration to a spherical atlas utilizing individual cortical folding patterns to match cortical geometry across subjects,50 parcellation of the cerebral cortex into units based on gyral and sulcal structure,42, 51 and creation of a variety of surface-based data including maps of curvature and sulcal depth. This method uses both intensity and continuity information from the entire three-dimensional magnetic resonance volume in segmentation and deformation procedures to produce representations of cortical thickness, calculated as the closest distance from the gray/white boundary to the gray/cerebrospinal fluid boundary at each vertex on the tessellated surface.39 The maps are created using spatial intensity gradients across tissue classes and are therefore not simply reliant on absolute signal intensity. The maps produced are not restricted to the voxel resolution of the original data and are thus capable of detecting submillimeter differences between groups. Procedures for the measurement of cortical thickness have been validated against histological analysis52 and manual measurements.53, 54 Freesurfer morphometric procedures have shown good test–retest reliability across scanner manufacturers and field strengths.45, 55
Genotyping
DNA was obtained from peripheral blood on the day of magnetic resonance imaging exams, according to the salting-out protocol56 and then genotyped for CACNA1C rs1006737 using real-time PCR allelic discrimination. PCR amplification for rs1006737 was performed in 5 μl reactions with 5 ng of template DNA, 1 × TaqMan Universal Master Mix (Applied Biosystems, Foster City, CA, USA), 1 × each primer and probe assay, and H2O. Thermal cycling consisted of initial denaturation for 10 min at 95 °C, followed by 40 cycles of denaturation at 95 °C for 15 s and annealing at 60 °C for 1 min. Fluorescence detection was performed in the annealing step. Amplification and allelic discrimination were performed on a 7500 Real-Time System (Applied Biosystems). Quality control of Real-time PCR results was done by direct sequencing on an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems). The genotype distribution was in (χ2=.97) Hardy–Weinberg equilibrium.
Statistical analysis
Statistical analyses were performed using Stata 13.1 (Statacorp, College Station, TX, USA). Continuous data were described as mean±s.d. if normally distributed. In all analysis, two-tailed values of P<0.05 were considered statistically significant. Our first statistical analysis consisted of a linear regression, in which, cortical thickness (left and right sFC, mOFC, cACC and rACC) was the dependent variable, whereas age was the main explanatory variable adjusted for gender. We executed this linear regression separately in two groups: carriers (A/G and A/A) and non-carriers (G/G) of allele A CACNA1C rs1006737. We chose this strategy because our main hypothesis was that the presence of the CACNA1C rs1006737 risk allele A could affect cortical thickness. After that, to investigate the influence of medications in our results, we executed another linear regression in which cortical thickness (left and right sFC, mOFC, cACC and rACC) was the dependent variable, whereas age was the main explanatory variable adjusted for gender and medication status (antidepressants, lithium, anticonvulsants and/or antipsychotics). The regression coefficients (coef) are expressed together with their 95% confidence intervals (CIs).
Results
Sociodemographic allele distribution information is given in Table 1. Carriers and non-carriers of the A allele did not differ for age, gender, education, dexterity, disease duration or intracranial volume (Table 1), but there was a difference regarding the rate of lithium and antipsychotics use.
Table 1. Sociodemographic, medication use and prefrontal cortical thickness measures according to the presence of A allele CACNA1C rs1006737.
|
BD |
|||
|---|---|---|---|
| rs1006737 | Without A (N=67) | With A (N=50) | Sig. (two-tailed) |
| Age | 32.7±8.7 | 31.5±10.2 | P=0.1 |
| Gender (female/male) | 49/18 | 29/21 | χ2=0.08 |
| Dexterity (n; right/left) | 58/9 | 42/8 | χ2=0.69 |
| Education (years) | 13.3±3.5 | 13.1±2.6 | P=0.3 |
| Illness duration (years) | 7.46±7.1 | 6.18±5.42 | P=0.35 |
| Lithium use (yes/no) | 44/23 | 42/8 | χ2=0.02 |
| Anticonvulsants use (yes/no) | 23/44 | 14/36 | χ2=0.46 |
| Antipsychotics use (yes/no) | 30/37 | 14/36 | χ2=0.06 |
| Antidepressants use (yes/no) | 10/57 | 4/46 | χ2=0.25 |
| Intracranial volume (mean±s.d.)a | 1 319 411±279 256 | 1 317 830±209 098 | P=0.26 |
| Right mOFC (mean±s.d.)a | 2.48±0.16 | 2.54±0.21 | P=0.01 |
| Right sFC (mean±s.d.)a | 2.52±0.15 | 2.56±0.18 | P<0.001 |
| Right rACC (mean±s.d.)a | 2.97±0.18 | 3.01±0.26 | P=0.08 |
| Right cACC (mean±s.d.)a | 2.63±0.24 | 2.70±0.29 | P=0.86 |
| Left mOFC (mean±s.d.)a | 2.41±0.16 | 2.51±0.16 | P=0.005 |
| Left sFC (mean±s.d.)a | 2.51±0.15 | 2.56±0.20 | P<0.001 |
| Left rACC (mean±s.d.)a | 2.98±0.19 | 2.98±0.22 | P=0.009 |
| Left cACC (mean±s.d.)a | 2.65±0.25 | 2.66±0.29 | P=0.11 |
Abbreviations: BD, bipolar disorder; cACC, caudal anterior cingulate cortex; mOFC, medial orbitofrontal cortex; rACC, rostral anterior cingulate cortex; sFC, superior frontal cortex.
Mean and s.d. without adjust for age and gender.
CACNA1C A allele carriers (n=50) exhibited greater left mOFC thickness (coef=0.08, 95% CI=0.02, 0.14; P=.003) compared to non-carriers (n=67). No difference in right mOFC thickness was found between carriers and non-carriers of A (coef=0.46, 95% CI=−0.01, 0.11, P=0.16). There was no difference between carriers and non-carriers of A in sFC, cACC and rACC cortical thickness (left or right), after controlling for age and gender.
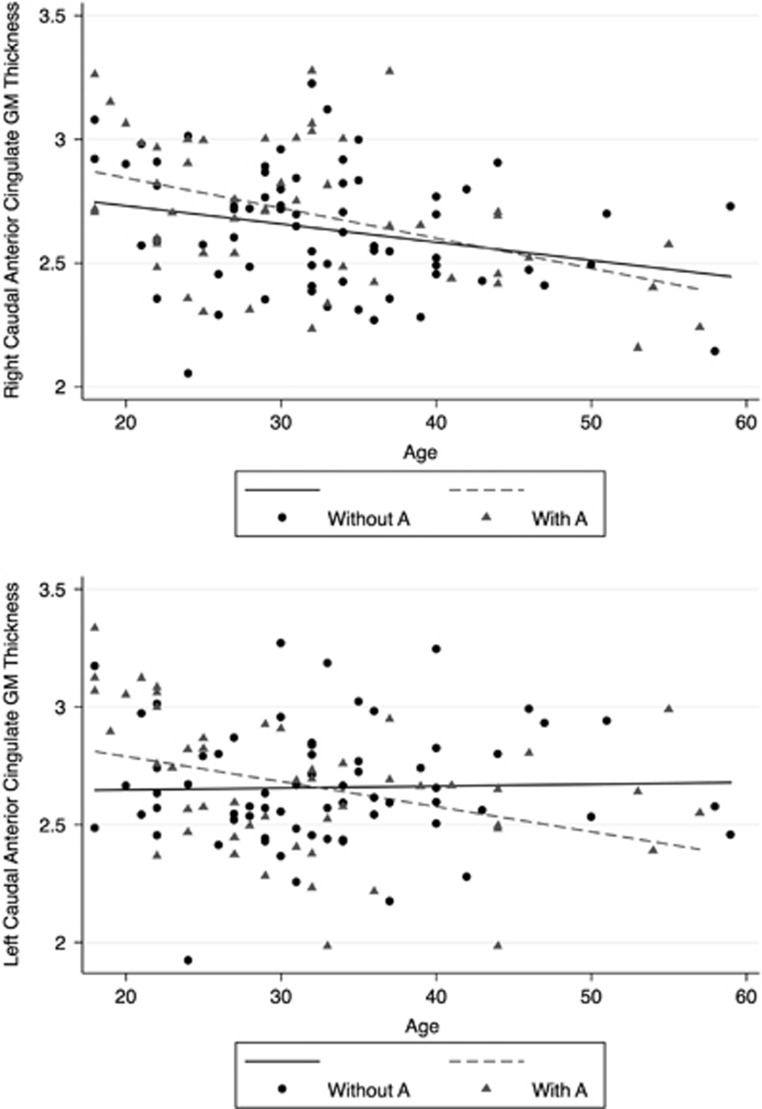
Subsequently, we investigated whether the CACNA1C A allele influenced the association between age and cortical thickness. Left cACC thickness negatively correlated with age in A carriers (coef=−0.012, 95% CI= −0.019, −0.004; P=.004) but not in non-carriers (coef=0.001, 95% CI=−0.007, 0.008; P=0.87; Figure 1). A negative correlation between age and cACC was detected for the right cACC, regardless of CACNA1C A status (A carriers (coef=−0.012, 95% CI=−0.018, −0.006; P<.001); non-carriers (coef=−2.2, 95% CI=−0.014, −0.0007; P=0.003)).
Figure 1.
Impact of age on cACC thickness in BD by rs1006737 A allele (corrected by gender and medication status). Right cACC A carriers (P=0.003) and A non-carriers (P<0.001). Left cACC A carriers (P=0.004); A non-carriers (P=0.87). BD, bipolar disorder; cACC, caudal anterior cingulate cortex.
Finally, we executed a linear regression controlling for medication status (use of antidepressants, anticonvulsants, lithium and/or antipsychotics). Results revealed that among allele A carriers the left cACC cortical thickness remained negatively influenced by age (coef=−0.011, 95% CI=−0.019, −0.004; P=0.004) and none of the four classes of medications demonstrated to influence the result. Among allele A non-carriers, there was no impact of age on left cACC cortical thickness (coef=−0.0001, 95% CI=−0.006, 0.006; P=0.97), but we observed a positive influence of lithium use (coef=0.14, 95% CI=0.004, 0.28; P=0.04) and a negative influence of antipsychotics (coef=−0.15, 95% CI=−0.29, −0.028; P=0.0018). The negative association between age and cortical thickness on the right cACC regardless status of A allele CACNA1C rs1006737 remained unchanged after controlling for medication status.
In the sFC, mOFC and rACC (left or right), a negative correlation was observed between age and cortical thickness, regardless of CACNA1C rs1006737 A status or medication use (Supplementary Figure 1).
Discussion
This study reports two main findings: (1) the influence of the rs1006737 A allele on cortical thickness; and (2) the influence of rs1006737 A allele on the correlation between cortical thickness and age. Euthymic BD I patients who were carriers of the CACNA1C rs1006737 A allele had greater left mOFC thickness compared to non-carriers. Moreover, the risk allele A was selectively associated with left cACC cortical thinning with increasing age, whereas no age-associated cortical thinning was observed in non-carriers.
Our results showing that A allele was associated with increased cortical thickness in left mOFC corroborate previous literature reporting that being a carrier of this allele is associated with greater volume or thickness of several brain structures compared to non-carriers in BD patients and HCs. The impact of the CACNA1C A allele on brain volume in BD type I has previously been evaluated in BD; two studies reported negative results,32, 57 whereas two reported increased volume associated with the A allele.1, 6 Perrier et al.1 reported that A carriers had increased GM density in the right amygdala and right hypothalamus in a sample of 41 BD euthymic type I patients compared to 50 HCs. In another study, Frazier et al.6 (BD type I= 96) reported that A allele carriers had larger bilateral caudate, insula, globus pallidus, frontal pole and nucleus accumbens volumes. The only study investigating the impact of CACNA1C on cortical thickness examined 121 BD patients (71 BD type I, 45 BD type II and 5 NOS) and 219 HC using a 1.5 T scanner and reported no effect of CACNA1C rs1006737 on frontal, parietal, temporal or total cortical thickness.32 To our knowledge, our study was the first to exclusively investigate BD type I during euthymia in a group of BD type I patients. We also used a 3 T scanner and focused only on mPFC thickness.
We also report that the CACNA1C A allele influences age-related changes in the mPFC in BD, specifically in the cACC. Allele A carriers demonstrated to have age-related cortical thinning in the left cACC, regardless of medication status. Curiously, the group of subjects not carrying allele A did not have age-related cortical thinning in this region but demonstrated to suffer from a negative impact of antipsychotics and a positive impact of lithium on the left cACC cortical thickness. Cortical thinning is the major manifestation of age-related changes in brain morphology and have been consistently described in both post-mortem and magnetic resonance imaging studies.58 These changes include reduction in total brain weight and cortical thickness, as well as gyral atrophy.58 Some studies have suggested that changes occur in association areas earlier with lesser changes taking place in primary sensory regions later.59 Moreover, these morphological alterations may be occur faster in particular areas of the cortex such as the PFC.54, 60, 61, 62 Cortical thickness can be modulated by numerous factors, including the number, size and myelination of neurons in the cortical columns.63, 64 For instance, neuronal survival and dendritic volume could be promoted in the adult brain by neurotrophic factors, such as brain-derived neurotrophic factor and glutamatergic signaling via N-methyl-d-aspartate receptors,65, 66 which have been reported to be altered in BD.67, 68 Therefore, the fact that L-type voltage-gated calcium channels control the influx of calcium into neurons and activate calcium-dependent processes, with a key role in neuron plasticity,23 might indicate why CACNA1C influences brain morphology. Moreover, it has been described that the use of antipsychotic medication has a negative impact on cortical thickness,69, 70 whereas lithium is reported to be associated with increases in cortical and subcortical GM volume,71, 72 which is in accordance to our results in non-carriers of CACNA1C allele A rs1006737.
The mechanism by which the A allele influences brain morphology is not fully elucidated, but some data indicate that this may occur through direct regulation of calcium channels activity, neuroplasticity and apoptosis regulation. These molecular changes may result in increased brain volume,16, 31 reduced emotional and cognitive processing,27, 73 increased brain activation signals during cognitive processing,27, 73 and decreased regional connectivity.16 The CACNA1C A/A genotype has also been associated with greater CACNA1C messenger RNA expression in the dorsolateral prefrontal cortex than G/G or A/G genotypes.27 Another recent study reported that BD patients carrying the CACNA1C allele A had higher levels of intracellular calcium compared to HCs.28 Similarly, Yoshimizu et al.74 reported elevated CACNA1C messenger RNA and greater calcium current density in neurons derived from allele A homozygotes compared to heterozygotes and non-risk (G/G) homozygotes. Higher intracellular calcium causes erratic activation of Ca2+-dependent pathways that are normally latent or operate at low levels, causing metabolic imbalances and eventual cell death.75 For example, acute raises in intracellular Ca2+ may over-activate proteases, lipases, phosphatases and endonucleases that either directly damage cell structure or induce the formation of oxidative-free radicals that mediate cell death.76 Taken together, these data support the view that higher activation or larger volumes of brain structures are not synonyms of better functioning. Thus, in a theoretical model, having the CACNA1C A allele would generate neurons with higher intracellular calcium levels, higher excitability and higher activation of calcium-dependent intracellular cascades. This phenomenon in BD patients, that per se have intrinsically higher activation of the glutamatergic system,77, 78 generates a double risk model for neuron hyperactivation based on glutamate and CACNA1C. Thus, the implications for cognition and age-related cortical thinning fit both models.
In summary, the present findings support a key role for the CACNA1C gene polymorphism rs1006737 A allele, previously reported to influence brain morphology and cognition, in the modulation of age-related cortical atrophy in cACC in BD. Our data reinforce the association between CACNA1C and morphological changes probably caused by alterations in neuroplasticity and neuron excitability patterns. Further studies investigating the link between the CACNA1C rs1006737 genotype and the cognitive/brain-morphological phenotype in BD type I are warranted. Moreover, future studies should investigate the rationale for L-type calcium channel antagonists as potential agents for preventing cognitive decline in BD type I patients carrying the CACNA1C A allele.
Acknowledgments
This study was supported by Sao Paulo Research Foundation (FAPESP) grant numbers 2012/23796-2 and 2010/18672-7. We thank LIM23 and AMJE (statistical analysis) for their support. This trial is registered on ClincalTrials.gov: NCT01237158.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
The authors declare no conflict of interest.
Supplementary Material
References
- Perrier E, Pompei F, Ruberto G, Vassos E, Collier D, Frangou S. Initial evidence for the role of CACNA1C on subcortical brain morphology in patients with bipolar disorder. Eur Psychiatry 2011; 26: 135–137. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res 2000; 126: 413–431. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry 2003; 54: 504–514. [DOI] [PubMed] [Google Scholar]
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann NY Acad Sci 2001; 935: 107–117. [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain 1995; 118: 279–306. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Youngstrom EA, Frankel BA, Zunta-Soares GB, Sanches M, Escamilla M et al. Candidate gene associations with mood disorder, cognitive vulnerability, and fronto-limbic volumes. Brain Behav 2014; 4: 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Blond BN, van Dyck LI, Spencer L, Wang F, Blumberg HP. Trait and state corticostriatal dysfunction in bipolar disorder during emotional face processing. Bipolar Disord 2012; 14: 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross LC, Thompson PM, Sugar CA, Madsen SK, Shen JK, Penfold C et al. Investigation of cortical thickness abnormalities in lithium-free adults with bipolar I disorder using cortical pattern matching. Am J Psychiatry 2011; 168: 530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr 2008; 13: 663–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kalmar JH, He Y, Jackowski M, Chepenik LG, Edmiston EE et al. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry 2009; 66: 516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MAR, O'Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet 2008; 40: 1056–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychiatric GWAS Consortium Bipolar Disorder Working Group. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet 2011; 43: 977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EK, Grozeva D, Jones I, Jones L, Kirov G, Caesar S et al. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol Psychiatry 2010; 15: 1016–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimm M, Kircher T, Kellermann T, Markov V, Krach S, Jansen A et al. Effects of a CACNA1C genotype on attention networks in healthy individuals. Psychol Med 2011; 41: 1551–1561. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Shen Q, Xu Z, Chen M, Cheng L, Zhai J et al. The effects of CACNA1C gene polymorphism on spatial working memory in both healthy controls and patients with Schizophrenia or bipolar disorder. Neuropsychopharmacology 2012; 37: 667–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, McIntosh AM, He Y, Gelernter J, Blumberg HP. The association of genetic variation in CACNA1C with structure and function of a frontotemporal system. Bipolar Disord 2011; 13: 696–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke B, Vasquez AA, Veltman JA, Brunner HG, Rijpkema M, Fernández G. Genetic variation in CACNA1C, a gene associated with bipolar disorder, influences brainstem rather than gray matter volume in healthy individuals. Biol Psychiatry 2010; 68: 586–588. [DOI] [PubMed] [Google Scholar]
- Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 2000; 10: 206–219. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 2008; 13: 829–833–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace A, Thompson WK, Zhou D, Almeida JRC, Hassel S, Klein CR et al. Abnormal left and right amygdala-orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biol Psychiatry 2010; 67: 422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Whitfield-Gabrieli S, Shinn AK, Gabrieli JDE, Nieto Castañón A, McCarthy JM et al. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology 2011; 36: 2009–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD et al. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord 2012; 14: 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell 2007; 131: 1047–1058. [DOI] [PubMed] [Google Scholar]
- Tong G, Shepherd D, Jahr CE. Synaptic desensitization of NMDA receptors by calcineurin. Science 1995; 267: 1510–1512. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Carnahan J, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science 1994; 263: 1618–1623. [DOI] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 1998; 20: 709–726. [DOI] [PubMed] [Google Scholar]
- Bigos KL, Mattay VS, Callicott JH, Straub RE, Vakkalanka R, Kolachana B et al. Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch Gen Psychiatry 2010; 67: 939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Green M, Warsh JJ. CACNA1C SNP rs1006737 associates with bipolar I disorder independent of the Bcl-2 SNP rs956572 variant and its associated effect on intracellular calcium homeostasis. World J Biol Psychiatry 2016; 17: 525–534. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Björk C, Pawitan Y, Cannon TD, Sullivan PF et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet 2009; 373: 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localizadon of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci 1993; 5: 162–176. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Ruberto G, Vassos E, Tatarelli R, Girardi P, Collier D et al. Effects of the CACNA1C risk allele for bipolar disorder on cerebral gray matter volume in healthy individuals. Am J Psychiatry 2009; 166: 1413–1414. [DOI] [PubMed] [Google Scholar]
- Tesli M, Egeland R, Sønderby IE, Haukvik UK, Bettella F, Hibar DP et al. No evidence for association between bipolar disorder risk gene variants and brain structural phenotypes. J Affect Disord 2013; 151: 291–297. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JB. Structured Clinical Interview for DSM-IV AxisIDisorders SCID-I. American Psychiatric Press: Washington, DC, USA, 1996. [Google Scholar]
- DSM-IV PATFODiagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. American Psychiatric Publishing, Inc.: Washington, DC, 2000. [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133: 429–435. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatr 1960; 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999; 9: 179–194. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci 1993; 5: 162–176. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000; 97: 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging 2001; 20: 70–80. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33: 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH et al. Automatically parcellating the human cerebral cortex. Cereb Cortex 2004; 14: 11–22. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 1999; 8: 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJW, Makris N, Ségonne F, Quinn BT et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage 2004; 23: S69–S84. [DOI] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage 2006; 32: 180–194. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage 2006; 30: 436–443. [DOI] [PubMed] [Google Scholar]
- Ségonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage 2004; 22: 1060–1075. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 1998; 17: 87–97. [DOI] [PubMed] [Google Scholar]
- Ségonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging 2007; 26: 518–529. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 1999; 9: 195–207. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31: 968–980. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH et al. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology 2002; 58: 695–701. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry 2003; 60: 878–888. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E et al. Thinning of the cerebral cortex in aging. Cereb Cortex 2004; 14: 721–730. [DOI] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 2012; 61: 1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen J, Samarut J, Hölttä E. A nontoxic and versatile protein salting-out method for isolation of DNA. BioTechniques 1994; 17: 316–322. [PubMed] [Google Scholar]
- Soeiro de Souza MG, Otaduy MCG, Dias CZ, Bio DS, Machado-Vieira R, Moreno RA. The impact of the CACNA1C risk allele on limbic structures and facial emotions recognition in bipolar disorder subjects and healthy controls. J Affect Disord 2012; 136: 370–376. [DOI] [PubMed] [Google Scholar]
- Kemper TL. Neuroanatomical and neuropathological changes during aging and in dementia. In: Albert ML, Knoepfel EJE (eds). Clinical Neurology of aging. Oxford University Press: New York, USA, 1994, pp 3–67.
- Flood DG, Coleman PD. Neuron numbers and sizes in aging brain: comparisons of human, monkey, and rodent data. Neurobiol Aging 1988; 9: 453–463. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR. Cerebral structure on MRI, Part I: localization of age-related changes. Biol Psychiatry 1991; 29: 55–67. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex 1997; 7: 268–282. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci 2003; 6: 309–315. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex 2009; 19: 2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science 1988; 241: 170–176. [DOI] [PubMed] [Google Scholar]
- Monfils M-H, VandenBerg PM, Kleim JA, Teskey GC. Long-term potentiation induces expanded movement representations and dendritic hypertrophy in layer V of rat sensorimotor neocortex. Cereb Cortex 2004; 14: 586–593. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Graham ME, Cambray-Deakin M. Neurotrophic effects of NMDA receptor activation on developing cerebellar granule cells. J Neurocytol 1993; 22: 689–695. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Soeiro-de-Souza MG, Richards EM, Teixeira AL, Zarate CA. Multiple levels of impaired neural plasticity and cellular resilience in bipolar disorder: Developing treatments using an integrated translational approach. World J Biol Psychiatry 2013; 15: 84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro-de-Souza MG, Dias VV, Figueira ML, Forlenza OV, Gattaz WF, Zarate CA et al. Translating neurotrophic and cellular plasticity: from pathophysiology to improved therapeutics for bipolar disorder. Acta Psychiatr Scand 2012; 126: 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smieskova R, Fusar-Poli P, Allen P, Bendfeldt K, Stieglitz RD, Drewe J et al. The effects of antipsychotics on the brain: what have we learnt from structural imaging of schizophrenia?--a systematic review. Curr Pharm Des 2009; 15: 2535–2549. [DOI] [PubMed] [Google Scholar]
- Lesh TA, Tanase C, Geib BR, Niendam TA, Yoon JH, Minzenberg MJ et al. A multimodal analysis of antipsychotic effects on brain structure and function in first-episode schizophrenia. JAMA Psychiatry 2015; 72: 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassi RB, Nicoletti M, Brambilla P, Mallinger AG, Frank E, Kupfer DJ et al. Increased gray matter volume in lithium-treated bipolar disorder patients. Neurosci Lett 2002; 329: 243–245. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Thompson PM, Dalwani M, Hayashi KM, Lee AD, Nicoletti M et al. Greater cortical gray matter density in lithium-treated patients with bipolar disorder. Biol Psychiatry 2007; 62: 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug A, Nieratschker V, Markov V, Krach S, Jansen A, Zerres K et al. Effect of CACNA1C rs1006737 on neural correlates of verbal fluency in healthy individuals. Neuroimage 2010; 49: 1831–1836. [DOI] [PubMed] [Google Scholar]
- Yoshimizu T, Pan JQ, Mungenast AE, Madison JM, Su S, Ketterman J et al. Functional implications of a psychiatric risk variant within CACNA1C in induced human neurons. Mol Psychiatry 2015; 20: 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler R, Tymianski M. Molecular mechanisms of calcium-dependent excitotoxicity. J Mol Med 2000; 78: 3–13. [DOI] [PubMed] [Google Scholar]
- Tymianski M, Tator CH. Normal and abnormal calcium homeostasis in neurons: a basis for the pathophysiology of traumatic and ischemic central nervous system injury. Neurosurgery 1996; 38: 1176–1195. [DOI] [PubMed] [Google Scholar]
- Yüksel C, Ongur D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry 2010; 68: 785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro de Souza MG, Salvadore G, Moreno RA, Otaduy MCG, Chaim KT, Gattaz WF et al. Bcl-2 rs956572 polymorphism is associated with increased anterior cingulate cortical glutamate in euthymic bipolar I disorder. Neuropsychopharmacology 2013; 38: 468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.