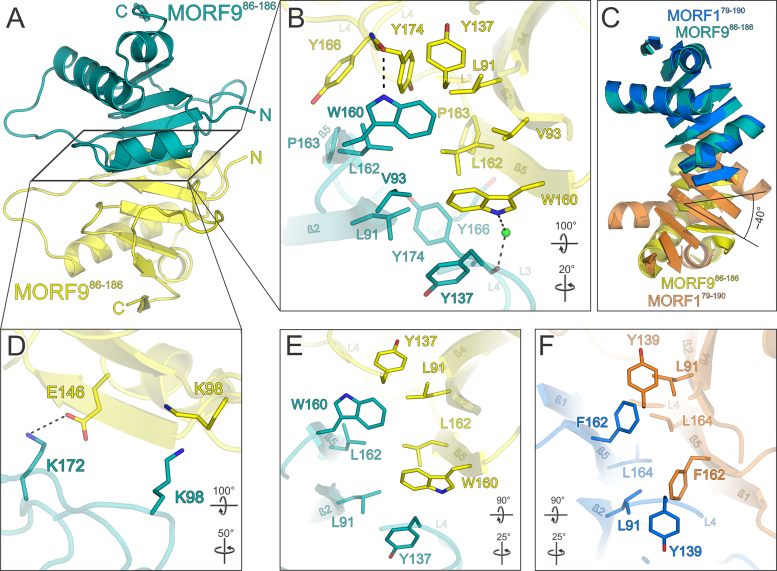
Figure 5.
Structure of the MORF986–186 MORF domain and its dimerization in relation to MORF1. (A) Overview of the MORF986–186 structure. A central MORF986–186 molecule (teal) forms a tight nearly symmetrical head-to-head dimer via a hydrophobic interaction surface with a second MORF986–186 molecule (yellow). (B) Close-up view of the main hydrophobic protein–protein interface of MORF986–186 shown in (A). (C) Superimposition of the MORF179–190 (marine blue and orange) and MORF986–186 (teal and yellow) dimers. Loops are omitted for clarity. The second MORF986–186 molecule (yellow) is rotated by an angle of ∼40° with respect to the respective MORF179–190 moiety (orange) as indicated for β-strand 4. (D) Close-up view of a peripheral MORF986–186 interface of the dimer shown in (A). (E and F) Comparison of the hydrophobic dimerization interfaces of MORF179–190 and MORF986–186. The selection of interacting residues is limited to positional or sequence identities shared by MORF1 and MORF9. Interacting residues are shown as sticks and colored by atom type. Carbon – as for the respective molecule; nitrogen – blue; oxygen – red; sulfur – yellow. Water oxygens are shown as green spheres. Dashed lines between atoms represent hydrogen bonds. Rotation symbols indicate views relative to Figure 2A.