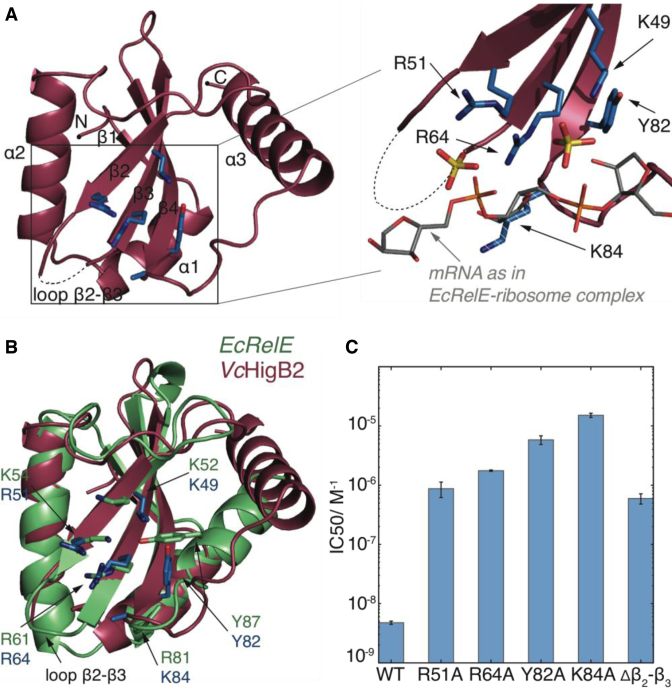
Figure 1.
The structure and activity of VcHigB2 toxin. (A) Left: the structure of the VcHigB2 toxin. Secondary structure elements and positively charged β2-β3 loop, which is missing from the model are indicated. Active site residues are shown as blue sticks. Right: detailed view of the active site residues, which coordinate two sulphate ions from the crystallization mixture (yellow). The mRNA (gray) as found in the EcRelE-ribosome structure (PDB ID: 4V7J) is superimposed for comparison. The side chain of Lys84 lacks clear electron density, but is modeled here in a likely position and shown as dashed stick. (B) Comparison of the active site of the VcHigB2 (red) and the EcRelE (green, PDB ID: 3KHA). VcHigB2 residues (blue sticks) that correspond to the active site residues of EcRelE (green sticks) are shown. (C) Concentrations of WT toxin and its variants to achieve half-maximal inhibition of the reporter protein synthesis in the in vitro activity assay.