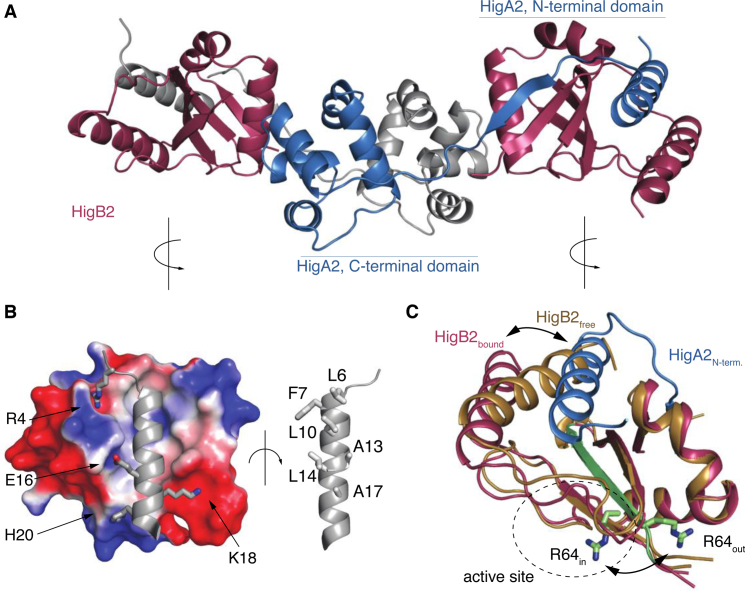
Figure 4.
Structure of the heterotetrameric VcHigBA2 complex. (A) Overall structure of the complex. The two VcHigB2 toxins are in red, the two chains of the antitoxin dimer are in gray and blue. (B) Interactions between the toxin and the antitoxin's N-terminal domain. The electrostatic surface of the VcHigB2 toxin was calculated with ABPS using default parameters (60). (C) Conformational changes in the VcHigB2 induced by binding to VcHigA2. The antitoxin N-terminal helix (blue) partially displaces the VcHigB2 C-terminal helix (toxin in its free state is shown in gold, toxin in the bound state in red) and shifts the register of the β-strand β3 (green). The two positions of the catalytically important Arg64 are shown in sticks.