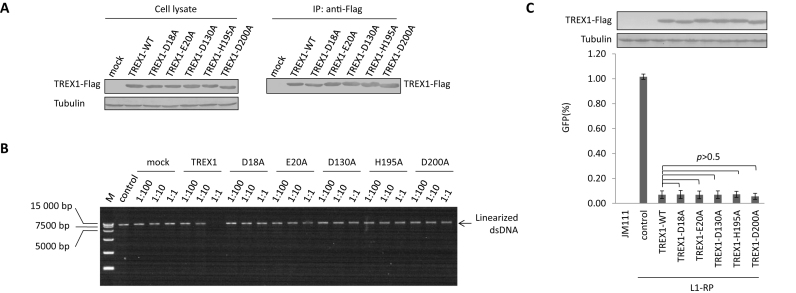
Figure 2.
An exonuclease-independent mechanism contributes to TREX1-mediated L1 suppression. (A) Western blotting results showing the protein levels of TREX1 in both cell lysates and co-IP eluates. Three micrograms of vectors expressing TREX1 or its DNase-defective mutants were transfected into HEK293T cells seeded on a 6-cm dish. At 48 h post-transfection, the cells were harvested for the IP assay shown in A and DNase activity assay shown in B. (B) Agarose electrophoresis results showing that wild-type TREX1, and not its exonuclease-deactivated mutants, can digest linearized DNA. M, DL15 000 DNA marker (Takara). Linearized L1-RP was used in place of VR1012 as the substrate to confirm that the tested mutants did not affect the L1 assay results by compromising exonuclease activity. The ratios shown are dilution rates of extract TREX1 proteins. (C) L1 assay results showing that exonuclease-deactivated TREX1 mutants maintain potency against L1 replication. A total of 450 ng of VR1012 empty vector or vectors expressing TREX1 or its DNase-defective mutants were co-transfected with 2 μg L1-RP into HEK293T cells seeded on a 12-well plate to examine potency against L1 retrotransposition. At 4 days post-transfection, EGFP-positive cells were determined by flow cytometry. JM111 was used as the negative control for flow cytometry gating, and VR1012 was the empty vector used as the negative control for TREX1 expression. The western blotting results show the expressed levels of TREX1 and its mutants. All the data shown in this figure are representative of at least three independent experiments. The error bars shown in C indicate the SD of three replicates within one experiment.