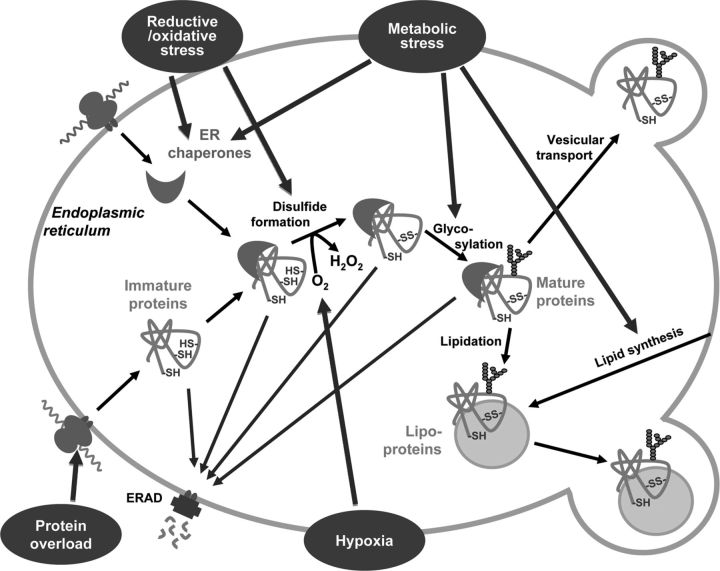
Figure 1.
ER stressors influence ER proteostasis. Proteins formed in the ER are folded and posttranslationally modified in the luminal compartment. Various cofactors are required for posttranslational modifications. After the synthesis of the polypeptide chain by the ribosomes, oxygen is needed for disulfide formation, various carbohydrates for protein glycosylation, and lipids for lipidation. During the folding process, the proteins are accompanied by different ER chaperones in the luminal compartment. Increased demand for protein folding due to protein overload or various other ER stressors, eg, redox stress, hypoxia, and metabolic stress, can change supplies of cofactor, thereby influencing the folding process. Improperly folded immature proteins can be degraded by the ERAD, whereas mature proteins are exported to secretory vesicles.