Abstract
The pituitary is a complex gland comprising different cell types each secreting specific hormones. The extensive network of signaling molecules and transcription factors required for determination and terminal differentiation of specific cell types is still not fully understood. The SRY-like HMG-box (SOX) transcription factor Sox4 plays important roles in many developmental processes and has two homologs in zebrafish, Sox4a and Sox4b. We show that the sox4b gene is expressed in the pituitary anlagen starting at 24 h after fertilization (hpf) and later in the entire head region including the pituitary. At 48 hpf, sox4b mRNA colocalizes with that for TSH (tshβ), glycoprotein subunit α (gsuα), and the Zn finger transcription factor Gata2a. Loss of Sox4b function, using morpholino knockdown or expression of a dominant-negative Sox4 mutant, leads to a drastic decrease in tshβ and gsuα expression and reduced levels of gh, whereas other anterior pituitary gland markers including prl, slβ, pomc, and lim3 are not affected. Sox4b is also required for expression of gata2a in the pituitary. Knockdown of gata2a leads to decreased tshβ and gsuα expression at 48 hpf, similar to sox4b morphants. Injection of gata2a mRNA into sox4b morphants rescued tshβ and gsuα expression in thyrotrope cells. Finally, sox4b or gata2a knockdown causes a significant decrease of gonadotropin expression (lhβ and fshβ) at 4 d after fertilization. In summary, our results indicate that Sox4b is expressed in zebrafish during pituitary development and plays a crucial role in the differentiation of thyrotrope and gonadotrope cells through induction of gata2a expression in the developing pituitary.
In the last decades, knowledge concerning development of the pituitary gland has been gained from identification of genes involved in human congenital pituitary hormone deficiency, a pathology characterized by hypopituitarism (1). Also, spontaneous mutations and reverse genetics in murine models revealed that normal anterior pituitary formation is dependent upon a complex genetic cascade of signaling molecules and transcription factors (1–3). More recently, studies in mammals have been complemented by those in zebrafish that might prove useful to uncover thus far unidentified mechanisms and regulators of pituitary development and to better understand the evolution of this important gland.
The basic mechanisms of anterior pituitary development appear to be conserved between fish and mammals. The mature gland is formed of several cell types, each secreting a specific hormone (4): somatotropes producing GH, lactotropes secreting prolactin (Prl), thyrotropes synthesizing TSH, and gonadotropes producing LH and FSH. The glycoprotein α-subunit (Gsuα) is expressed in thyrotropes and gonadotropes, where it associates respectively with Tshβ or Lhβ and Fshβ. ACTH and MSH, both proteolytically cleaved from the same precursor proopiomelanocortin, are secreted by, respectively, corticotropes and melanotropes. An additional pituitary hormone is present in teleosts, somatolactin, closely related to Prl and Gh. Two sl genes have been identified in zebrafish that are expressed in specific domains of the adult gland (5). One of them, slβ, was shown to be expressed in wild-type (wt) zebrafish from 23 h after fertilization (hpf) onward (6).
Like in other vertebrates, the initial steps of zebrafish anterior pituitary formation start when gastrulation is completed at approximately 10 hpf. Specific transcription factors gradually restrict pituitary cell development and promote their differentiation into specific cell types. At this phase of development, the neural plate is bordered caudally by the neural crest and rostrally by the preplacodal ectoderm (PPE). Hedgehog signaling from the ventral forebrain specifies the median region of the PPE to form the pituitary placode (7). At this early stage, the PPE is characterized by the expression of multiple marker genes, such as eyes absent (eya1) and sine oculis (six1) (8), the bicoid-related paired-like homeobox gene pitx3 or the dlx3b gene (7, 9). The first anterior pituitary-specific marker to come up during the mid-segmentation stage (18–20 hpf) is the Lim-domain homeobox gene lim3, which is expressed in all anterior pituitary precursor cells. Another early marker is the POU domain homeobox factor Pit1 (10, 11). In contrast to the pan-pituitary marker lim3, pit1 is activated in just a subset of anterior pituitary cells. In mouse, loss of Pit1 function leads to absence of gh- and prl-expressing cells and a trans-fating of thyrotropes to gonadotropes (10, 11). Zebrafish pit1 mutants appear to display a similar thyrotrope to gonadotrope trans-fating (11). Another factor specifically involved in thyrotrope and gonadotrope formation in the mouse is the Zn-finger transcription factor Gata2 (12). During normal thyrotrope development, Pit1 attenuates expression of the lhβ gene by binding and blocking the transcription factor Gata2 (12).
Transcription factors of the SRY-related HMG box (SOX) family are characterized by the presence of a highly conserved, DNA-binding HMG box domain. These factors are widely and dynamically expressed throughout embryogenesis and have been implicated in many developmental processes (13) ranging from early cell fate determination (14) to control of lens development (15–17) or chondrogenesis (18–20). Sox3 was the first member of this family to be associated with X-linked hypopituitarism in mice and humans (21, 22). It is expressed in the ventral diencephalon and in the infundibulum, adjacent to the Rathke's pouch, but not in the presumptive anterior pituitary (21–23). Sox2 was similarly found to be critical for the development of the hypothalamo-pituitary axis by maintaining a pool of undifferentiated cells in the pituitary (24, 25).
Sox4 is a member of the group C Sox proteins that contains a C-terminal transcription activation domain conserved within this group and has been involved in the development of various structures (26–30). Two Sox4 homologs are present in zebrafish, but only sox4b was shown to be expressed in pancreas and required for differentiation of glucagon-expressing cells (31), similar to the function of Sox4 in normal development of pancreatic islets in mouse (32). Recently, SOX4 was found to be one of the main transcription factors expressed in fetal pituitary in human (33). However, its function in the pituitary has not been investigated so far.
Here, we investigate the function of the zebrafish Sox4 homolog, Sox4b, in pituitary development. We show that sox4b is specifically expressed in the anterior pituitary primordium at 24 and 48 hpf, more specifically in gsuα-, gata2a- and tshβ-expressing cells. We show that sox4b is required for the differentiation of thyrotrope and gonadotrope cells, and finally, we demonstrate that gata2a expression is regulated by Sox4b in developing zebrafish pituitary.
Materials and Methods
Zebrafish maintenance, mutant lines, and microinjection
Zebrafish (Danio rerio) were maintained under standard conditions (34) in the GIGA-R zebrafish facility. Wild-type embryos from the AB strain were produced and staged according to Kimmel et al. (35). The pit1 mutant was previously described (8, 11, 36).
Antisense morpholinos (MO) (Gene Tools, Philomath, OR) were used. Standard control MO (coMO) 5′-CCTCTTACCTCAGTTACAATTTATA-3′ is known to have no target and no significant biological activity in zebrafish embryos; sox4bMO 5′-GACTCAGTCTGATTGCACACAGTCC-3′ and sox4bMO2 5′-TGCTGCTGGATCTCTGGAGCAT-3′, targeting the 5′-untranslated region of sox4b or the first 25 bases of the coding sequence, respectively, to block translation, were previously described (31) and were injected at an optimal dose of 7 ng/egg or 2 ng/egg, respectively; and gata2aMO 5′-CATCTACTCACCAGTCTGCGCTTTG-3′ is a splice blocking MO targeting the third exon/intron boundary of the gata2a gene (30) and was used at a dose of 0.5 ng/egg.
The mRNA coding for the dominant-negative mutant Sox4bΔC (37) or enhanced green fluorescent protein was injected at 0.2 ng/egg. To perform phenotypic rescue experiments, gata2a mRNA was synthesized and then injected into sox4b- or gata2aMO-injected or wt embryos at concentrations of 100 and 75 pg, respectively. MO were diluted in Danieau's buffer containing 0.5% rhodamine-dextran for checking the injection.
Microinjection was performed at the one-cell stage; the injected embryos were fixed at different stages in 4% paraformaldehyde and stored in 100% methanol before analysis.
Riboprobes and whole-mount in situ hybridization (WISH)
Single and double whole-mount and double-fluorescent in situ hybridizations were performed as previously described (37, 38) on wt and mutant embryos. Antisense RNA probes were prepared by transcribing linearized cDNA clones with SP6, T7, or T3 polymerase using digoxigenin labeling mix (Roche, Indianapolis, IN) or DNP-11-UTP ribonucleotides (TSAi Plus system; PerkinElmer, Norwalk, CT). The riboprobes used were lim3, gata2a, pit1, prl, gsuα, tshβ, gh and slβ (6, 11, 39–41) as described. The lhβ cDNA (438 bp) (accession no. HE608243) covering the partial coding region was obtained by performing RT-PCR on mRNA from 5-d-old [days after fertilization (dpf)] zebrafish larvae using the primers zfLHBfor3 (cagcctgctgagcaac) and zfLHBrev1 (cctctctctgggacatgcagaag) and contained three silent mutations relative to the reference lhβ sequence.
Fluorescence imaging
For confocal analysis, images were acquired using a Leica TCS SP2 inverted confocal laser microscope (Leica Microsystems, Heidelberg, Germany) equipped with one argon and two helium-neon lasers. Digitized images were acquired using a ×10 (NA 0.4) or ×63 (NA 1.4) Plan-Apo water-immersion objectives at 1024- × 1024-pixel resolution. The diameter of the pinhole was set up equal to the Airy unit. Series of optical sections were carried out to analyze the spatial distribution of fluorescence, and for each embryo, they were recorded with a Z-step ranging set to 2.0 μm. Images were acquired under identical conditions, and we ensured that the maximal fluorescence signal was not saturating the photomultiplier tubes. For multicolor imaging, fluorescein isothiocyanate was visualized by using an excitation wavelength of 488 nm, and the emission light was dispersed and recorded at 500–535 nm. Cy3 was detected by using an excitation wavelength of 543 nm and the 488/543 dichroic mirror, and the emission light was dispersed and recorded at 595–650 nm. The acquisition was set up to avoid any cross talk of the two fluorescence emissions. Captured images were exported as TIFF format files and further processed using Adobe Photoshop.
RNA extraction and reverse transcription
Total RNA was extracted from about 100 larvae per experiment using the RNeasy Mini kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. After extraction, the quality and concentration of total RNA was evaluated by electrophoresis on capillary gel and the ratio of absorbance at 260/280 nm by spectrophotometer. Synthesis of cDNA was performed from 1 μg total RNA, which was reverse transcribed using the transcriptor iScript cDNA synthesis kit (Bio-Rad, Nazareth, Belgium) according to the manufacturer's instructions.
Real-time PCR
Gene-specific oligonucleotide primers were developed using the Primer3 software and selected so as to span exon-exon junctions to avoid detection of genomic DNA (see Table 1 for primers used in quantitative RT-PCR) and synthesized by Eurogentec (Seraing, Belgium).
Table 1.
List of primers used for RT-PCR
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| tshβ | CTGTCAACACCACCATCTGC | GTGCATCCCCTCTGAACAAT |
| gsuα | TCACATCAGAAGCCACTTGC | GTGGCAGTCTGTGTGGTTGT |
| lim3 | AGGAGGACGGCATGGACA | ATCGGACATGGGCGGC |
| ef1α | ACATGCTGGAGGCCAGCTC | TACCCTCCTTGCGCTCAATC |
| pomc | AGCTCAGTGTTGGGAAAACG | GGTAGACGGGGGTTTCATCT |
| prl | ATGACAAAGACCAAGCCATG | GTTCTGGATGTGCCAGACTG |
| gh | CCTCTGTCGTTCTGCAACTC | ACTCCCAGGATTCAATGAGG |
| slβ | GTATATTCCTGGAGGGGCTG | ATTCACCAATGGCTCAATCC |
| lhβ | GCAGAGACACTTACAACAGCC | AAAACCAAGCTCTGGAGCAGCC |
| fshβ | CTGCAGATGAGGATGCGTGTGC | CTATGCTGGACAATGGATCG |
Real-time PCR was performed on an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA) with SYBR green fluorescent label. Samples (25 μl final volume) contained the following: 1× SYBR green master mix (Diagenode, Liège, Belgium), 150 nmol of each primer, and 1 μl of the reverse transcription reaction (1/20 of the total cDNA). Samples were run in duplicate in optically clear 96-well plates (ABgene, Epsom, UK). Cycling parameters were 50 C for 2 min, 95 C for 10 min, and then 40 cycles of 95 C for 15 sec and 60 C for 1 min. A melting temperature-determining dissociation step was performed at 95 C for 15 sec, 60 C for 15 sec, and 95 C for·15 sec at the end of the amplification phase. For analysis by endpoint PCR, the final products of the quantitative real-time RT-PCR (qRT-PCR) obtained after 40 PCR cycles were loaded on agarose gel for electrophoresis.
For the test of gonadotropin expression (lhβ and fshβ), thermal cycling was performed on an Applied Biosystems 7900 HT sequences detection system. The cDNA was used for qRT-PCR with the SensiMix SYBR Kit (Bioline, London, UK), containing SYBR green. The standard conditions were used with a modification in the elongation step: 62 C for 20 sec for lhβ and 62 C for 30 sec for fshβ were used.
No-template controls were run for all reactions, and all RNA preparations were subjected to sham reverse transcription to check for the absence of genomic DNA amplification. The relative transcript level of each gene was obtained by the 2−ΔΔCt method (42) and normalized relative to the housekeeping gene elongation factor1α (ef1α) with the program SDS version 2.2. Results are presented as percent expression in MO-injected embryos relative to clutchmate control embryos ± sd.
Statistical analysis
Statistical analysis was performed on raw data using one-way ANOVA. Significance was set at P < 0.01. Data from biological replicates were averaged and shown as mean normalized gene expression ± sd.
Results
Sox4b is expressed in the developing anterior pituitary
The expression pattern of sox4b mRNA in developing pituitary was determined by WISH experiments in zebrafish embryos. Starting at 24 hpf, sox4b mRNA was detected in the anterior border of the neural plate, corresponding to the early pituitary placode (Fig. 1A). At 26 hpf, sox4b expression is increased in the adjacent ventral diencephalon and predominant in the telencephalon (Fig. 1B), although at later stages (48 hpf), sox4b mRNA is detected in broad regions of the developing forebrain and midbrain and in a subpopulation of cells in the developing anterior pituitary (Fig. 1C). Double-fluorescent in situ hybridizations analyzed by confocal microscopy revealed that sox4b-positive cells in the ventral neural layer also express the pituitary precursor marker lim3 at 30 hpf, confirming that they are located in the anterior pituitary primordium (Fig. 1, D–G). Finally, at 48 hpf, a clear colocalization of sox4b mRNA was observed with tshβ mRNA (Fig. 1, H–K), suggesting that sox4b is expressed in thyrotrope cells.
Fig. 1.
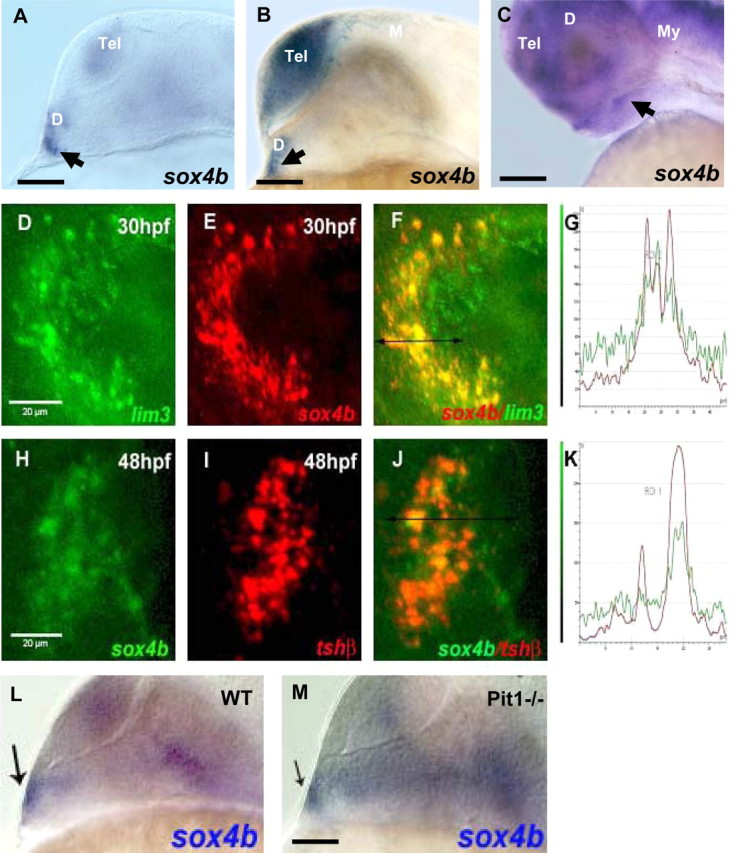
sox4b is expressed in the pituitary during zebrafish organogenesis. WISH was performed on wt zebrafish embryos using the sox4b antisense probe. A–C, Lateral views (anterior to the left, dorsal to the top) of wt embryos labeled with the sox4b probe (blue) by WISH. The arrowheads indicate the pituitary placode at 24 (A), 26 (B), and 48 (C) hpf, respectively. D–F and H–J, Analysis by double-fluorescent WISH (anterior to the left), with one z-plane of the confocal images shown; D–F, expression of sox4b (red, E) and the pan-adenohypophyseal marker lim3 (green, D) colocalize in the pituitary placode (F); G, fluorescence emission quantification in F; H–J, sox4b (green, H) and tshβ (red, I) are coexpressed in a subtype of pituitary cells (J); K, fluorescence emission quantification in J. In D–F and H–J, the ventral head regions were dissected before confocal microscopy. L and M, sox4b expression does not require pit1 at 24 hpf. Analysis of sox4b expression by WISH in wt and homozygous pit1 mutants. Lateral view, anterior to the left, the arrow indicates the label in the pituitary. Scale bars, 100 μm (A, B, C, L, and M) and 20 μm (D–J). D, Diencephalon; M, mesencephalon; My, myencephalon; Tel, telencephalon.
Sox4b expression was also assessed in the previously described pituitary mutant pit1 (8, 11, 36). Sox4b mRNA was readily detected in pituitaries of pit1 mutant embryos similar to their heterozygous or wt siblings at 24 hpf (Fig. 1, L and M).
Knockdown of Sox4b eliminates expression of tshβ and gsuα in the pituitary
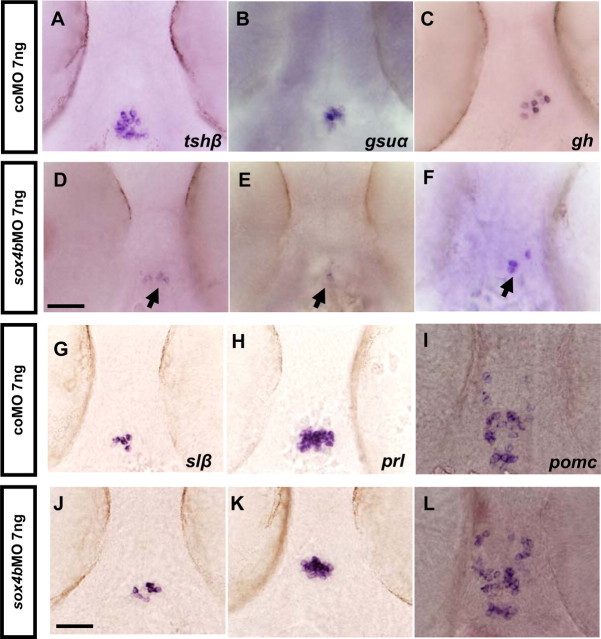
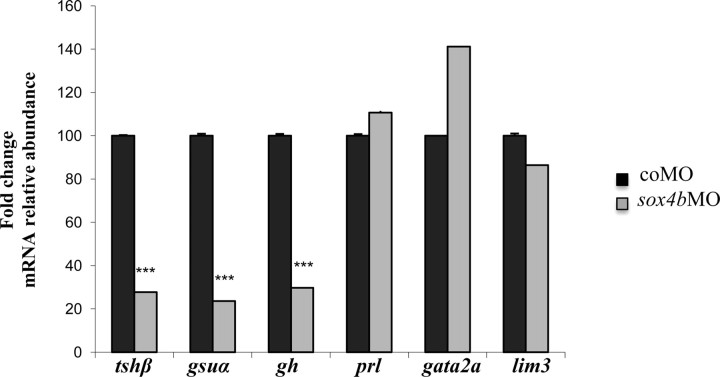
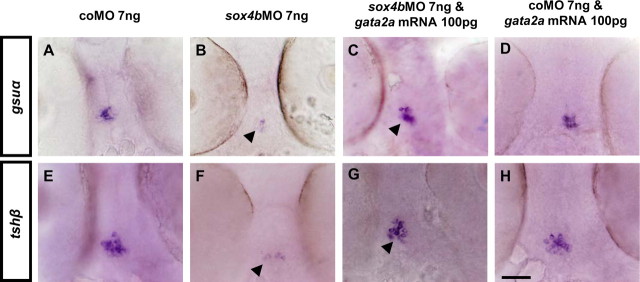
To investigate the function of Sox4b in pituitary development, we microinjected antisense MO oligonucleotides to ablate sox4b expression as previously described (31) and analyzed specific gene expression by WISH and by qRT-PCR in 48-hpf embryos. Control experiments checking pancreatic markers in the injected embryos confirmed the previously observed effect on glucagon expression (31) (Table 2). When the presence of the various pituitary hormone mRNA was investigated in these morphants (summarized in Table 2), we observed a drastic reduction of tshβ expression in sox4bMO-injected embryos at 48 hpf (Fig. 2, A and D). Similarly, the expression of gsuα was specifically reduced in sox4b morphants (Fig. 2, B and E), indicating that the two hormone subunits specific to thyrotrope cells are no longer expressed in these embryos. gh expression was reduced in 83% of the sox4bMO-injected embryos (Fig. 2, C and F), whereas slβ, prl, and pomc (Fig. 2, G–L) did not seem to be affected. Expression of gh, tshβ, and gsuα was also absent in 4-dpf sox4b morphants (Table 2) and in 48-hpf embryos previously injected with a different MO (sox4bMO2) against sox4b (Table 3). To confirm these results observed by in situ hybridization, qRT-PCR were performed using mRNA extracted from microinjected 48-hpf morphants and control embryos. The results confirmed the strong down-regulation of tshβ, gsuα, and gh expression (Fig. 3), whereas prl, the widely expressed lim3 factor (see also below) or the control mRNA ef1α (elongation factor 1) were not affected.
Table 2.
Sox4b regulates expression of tshβ, gsuα, and lhβ in zebrafish
| NI normal | coMO normal | sox4bMO absence | sox4bMO decrease | |
|---|---|---|---|---|
| Embryo, 48 hpf | ||||
| tshβ | 10/10 | 89/100 | 12/122 | 110/122a |
| gsuα | 10/10 | 38/40 | 0/38 | 38/38a |
| glucagon | 10/10 | 70/78 | 0/78 | 78/78a |
| slβ | 8/10 | 38/40 | 5/40 | 0/40 |
| pomc | 10/10 | 40/40 | 0/40 | 0/40 |
| lim3 | 10/10 | 38/38 | 2/40 | 0/40 |
| pit1 | 10/10 | 27/27 | 0/35 | 0/35 |
| gata2a | 10/10 | 40/40 | 38/40a | 0/40 |
| gh | 9/10 | 45/45 | 0/58 | 48/58a |
| prl | 10/10 | 50/50 | 0/50 | 0/50 |
| Larvae, 4 dpf | ||||
| lhβ | 10/10 | 50/50 | 0/50 | 28/50 |
| tshβ | 10/10 | 50/50 | 12/50a | 15/50a |
| gsuα | 10/10 | 50/50 | 0/50 | 30/50a |
| gh | 10/10 | 50/50 | 0/50 | 25/50a |
| prl | 10/10 | 50/50 | 0/50 | 0/50 |
Individual embryos, previously noninjected (NI) or injected with coMO or sox4bMO, were optically analyzed and compared for expression of different marker genes at 48 hpf and 4 dpf. The embryos were classified according to phenotype, and the number of each class is given relative to the total number of analyzed embryos for each condition. Absence indicates lost expression; decrease indicates that marker expression was reduced by more than 50%.
Significant at P < 0.001.
Fig. 2.
sox4b knockdown decreases tshβ, gsuα, and gh expression in 48-hpf embryos. Fertilized eggs were microinjected with sox4bMO and analyzed by WISH for pituitary hormone expression. A–C and G–I, Control embryos; D–F and J–L, sox4b morphants, ventral views (anterior to the top); both tshβ (D) and gsuα (E) expression is almost completely lost, whereas gh expression is decreased (F) and slβ, prl, and pomc are unchanged (J–L). Scale bars, 50 μm (A–L).
Table 3.
Individual embryos, injected with coMO or sox4bMO2, were optically analyzed and compared as in Table 1 at 48 hpf
| Embryos, 48 hpf | coMo 2 ng normal | sox4bMO2 2 ng absence | sox4bMO2 2 ng decrease |
|---|---|---|---|
| gsuα | 25/25 | 5/25a | 20/25a |
| tshβ | 25/25 | 0/20 | 20/25a |
| gh | 25/25 | 15/25a | 10/25a |
| prl | 25/25 | 0/25 | 0/25 |
Significant at P < 0.001.
Fig. 3.
qRT-PCR analysis of mRNA extracted from control and sox4b morphants. Total RNA was extracted from 48-hpf embryos injected with coMO or sox4bMO, and specific mRNA levels were determined by qRT-PCR. mRNA levels in control embryos were arbitrarily set to 100 for each primer pair and shown as mean relative levels ± se. The results shown are representative of three independent experiments for each mRNA. ***, Significant at P < 0.001.
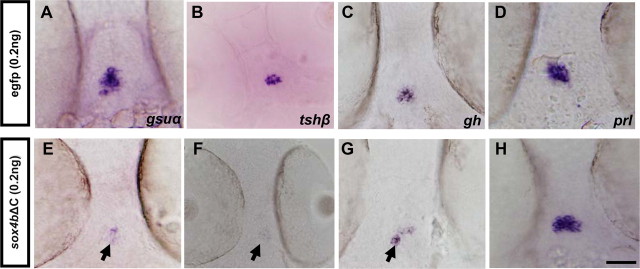
To confirm the specificity of the effects observed by MO injection, we abolished Sox4b function by microinjecting an mRNA coding for a Sox4b dominant-negative mutant, lacking its transactivation domain (sox4bΔC) (31). Injection of sox4bΔC mRNA into wt embryos also resulted in a decrease of gsuα, tshβ, and gh expression, whereas prl is not affected (Fig. 4), thus confirming the specificity of the defects observed upon MO injection.
Fig. 4.
Expression of the dominant-negative Sox4bΔC mutant decreases thyrotrope gene expression. sox4bΔC mRNA or egfp mRNA was microinjected into fertilized eggs, and WISH experiments were performed to detect gsuα (A and E), tshβ (B and F), gh (C and G), or prl (D and H) expression at 48 hpf. Ventral views (anterior to the top) are shown. Scale bar, 50 μm. Arrowheads in E–G point to the decreased expression in thyrotrope cells.
In conclusion, our results indicate that sox4b knockdown specifically affects expression and differentiation of thyrotrope and somatotrope cells.
Sox4b is required for the expression of gata2a specifically in the pituitary
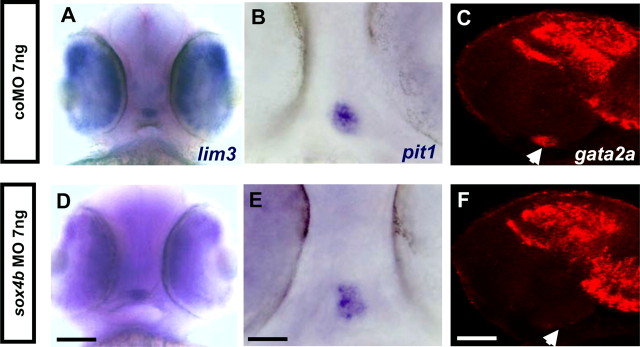
To shed some light on the molecular mechanisms involved in Sox4b function in the pituitary, we tested for the expression of known transcription factors in sox4b morphants. The pan-pituitary marker lim3 was not affected upon sox4b knockdown (Fig. 5, A and D, and Table 2). One of the factors required for thyrotrope differentiation is the pituitary-specific, POU-homeodomain factor Pit1 (10). Zebrafish pit1 mutants were shown to be deficient in prl, gh, slβ, and tshβ expression (6, 11). At 48 hpf, sox4bMO-injected embryos displayed unaltered pit1 expression in a frontal view (Fig. 5, B and E).
Fig. 5.
sox4b knockdown leads to decreased expression of gata2a. Fertilized eggs were microinjected with sox4bMO and analyzed by WISH for expression of lim3 and pit1 or by single fluorescent whole-mount in situ hybridization for gata2a (red) in the embryos at 48 hpf. A–C, Control; D–F, sox4bMO-injected embryos; A and D, ventral view (anterior to the top) (scale bar, 100 μm); B and E, ventral view (anterior to the top) (scale bar, 50 μm); C and F, z-plane confocal image lateral view (anterior to the left) (scale bar, 60 μm). Arrowheads in C and F point at the position of gata2a expression in the pituitary.
Another factor specifically involved in pituitary thyrotrope formation in mouse is the Zn-finger transcription factor Gata2 (12, 43). Although gata2a is widely expressed in the head region of the developing embryos at 48 hpf, we could clearly observe that its weak expression in the pituitary of coMO-injected embryos was completely absent in sox4b morphants (Fig. 5, C and F). Although qRT-PCR cannot directly assess the amount of gata2a or lim3 expression in the pituitary due to their wider expression in the head region, it is noteworthy that the overall amount of gata2a and lim3 mRNA was unchanged (or slightly increased) in the sox4b morphants (Fig. 3).
In conclusion, our data indicate that sox4b knockdown specifically affects the expression of gata2a in the developing pituitary and blocks differentiation of thyrotrope cells.
Sox4b and gata2a are specifically coexpressed in the anterior pituitary
Expression of gata2a was clearly observed in the pituitary at 48 hpf (Fig. 6A). Relative to the pomc-expressing corticotropes (Fig. 6B) and the prl-expressing lactotropes (Fig. 6C) located at the anterior border of the pituitary, gata2a-expressing cells are detected in a posterior domain. Double-fluorescent in situ hybridization revealed that gata2a is clearly coexpressed with gsuα (Fig. 6, D–G). Similarly, gata2a mRNA is colocalized with sox4b mRNA (Fig. 6, H–K) and is also present in tshβ-expressing cells, whereas some gata2a-positive cells did not express tshβ (Fig. 6, L–N), probably representing gonadotrope precursor cells. No colocalization was observed between gata2a and gh mRNA (Fig. 6, O–Q).
Fig. 6.
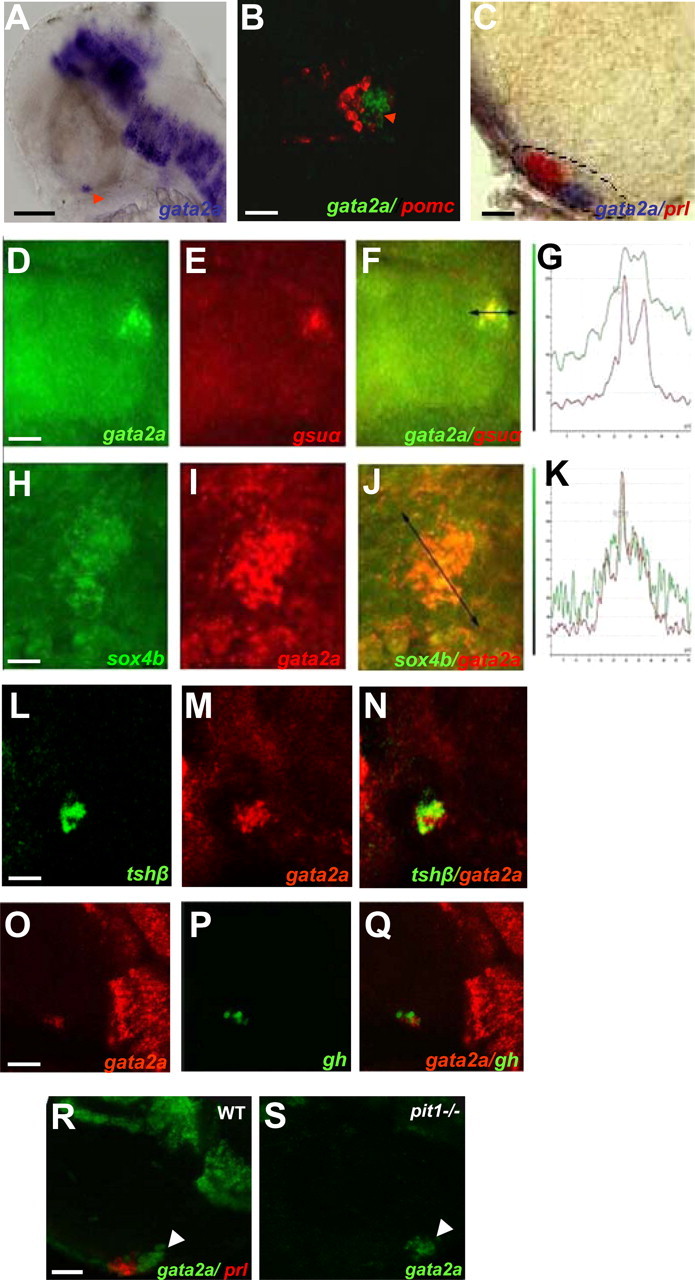
gata2a mRNA is detected in sox4b- and gsuα-expressing cells in 48-hpf embryos. WISH was performed on wt zebrafish embryos using the gata2a antisense probe. A and C, Lateral view (anterior to the left); B, ventral view (anterior to the top). Scale bars, 100 μm (A), 45 μm (B), and 40 μm (C). Arrowhead in A points at gata2a expression in the pituitary. A, Single-probe gata2a (blue); B, double-fluorescent WISH showing gata2a (green) and pomc (red) mRNA (z-plane confocal image); C, double WISH showing gata2a (blue) and prl (red) mRNA; the ventral head regions were dissected before microscopy. D–N, Double-fluorescent WISH showing coexpression as follows: D-G, of gata2a (green, D) and gsuα (red, E); F, overlay of D and E; G, fluorescence emission quantification in F. H-K, sox4b (green, H) and gata2a (red, I); J, overlay of H and I; K, fluorescence emission quantification in J. L–N, tshβ (green, L) and gata2a (red, M); N, overlay of tshβ and gata2a expression; D–N, z-plane confocal image, ventral views (anterior to the left). O–Q, Z-plane of the confocal images, lateral view (anterior to the left); gata2a (red, O) and gh (green, P); Q, overlay of gata2a and gh expression. R and S, gata2a expression in wt and pit1 mutants (pit1-/-) are shown in upper right corner in embryos at 48 hpf. Analysis by double-fluorescent WISH, lateral view (anterior to the left), one Z-plane of the confocal images is shown; gata2a (green, R and S) and prl (red, R) is shown, the arrowhead points to gata2a expression in the pituitary (R and S). Scale bars, 40 μm (D–F, L–N, R, and S), 20 μm (H–J), and 60 μm (O–Q).
Expression of gata2a was also tested by double in situ hybridization in the previously described pit1 mutant (8, 11, 36). We observed that expression of gata2a was similar to wt or heterozygous siblings at 48 hpf (Fig. 6, R and S).
Depletion of gata2a leads to loss of thyrotrope cells
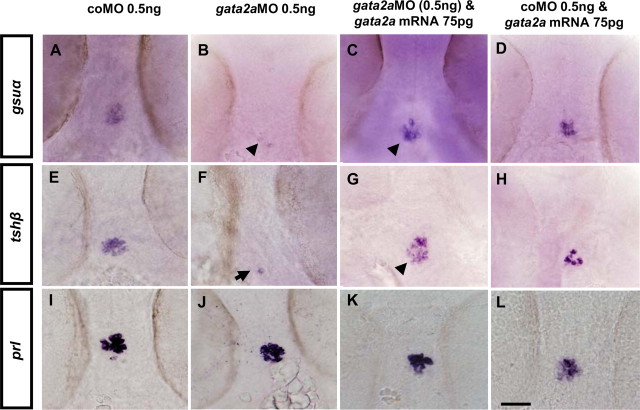
To compare the effects of gata2a and sox4b depletion in developing embryos, we microinjected gata2a MO (37) into fertilized eggs and analyzed hormone expression in 48-hpf embryos. Expression of tshβ and gsuα were drastically reduced in gata2a morphants relative to control MO-injected embryos (Fig. 7, A, B, E, and F), whereas prl expression was not affected (Fig. 7, I and J) at 48 hpf. When we coinjected mRNA coding for gata2a together with gata2a MO, expression of both tshβ and gsuα was clearly rescued (Fig. 7, C and G), whereas injection of gata2a mRNA alone had no effect (Fig. 7, D and H).
Fig. 7.
Thyrotrope expression depends on gata2a expression. WISH analysis for expression of tshβ, gsuα, and prl in 48-hpf embryos previously microinjected with (A, E, and I) coMO; (B, F, and J) gata2aMO, (C, G, and K) gata2aMO and gata2a mRNA or (D, H, and L) coMO and gata2a mRNA. A–L, Ventral views (anterior to the top). Scale bar, 50 μm.
Collectively, our results show that the defects due to gata2a depletion are very similar to those in sox4b morphants, suggesting that the two factors are components of a common pathway.
Exogenous Gata2a rescues Sox4b depletion in the pituitary
To understand the relationship between Gata2a and Sox4b in the regulatory pathways, we next asked whether ectopic expression of gata2a could restore expression in sox4b-depleted embryos.
Injection of sox4bMO resulted in a decrease of tshβ and gsuα expression, as expected, whereas coinjection of gata2a mRNA together with sox4bMO completely restored this expression at 48 hpf (Fig. 8).
Fig. 8.
Expression of exogenous Gata2a rescues the pituitary defects in sox4b morphants. WISH analysis was performed for expression of tshβ and gsuα in 48-hpf embryos previously microinjected with coMO (A and E), sox4bMO (B and F), sox4bMO and gata2a mRNA (C and G), or coMO and gata2a mRNA (D and H). A–H, Ventral views (anterior to the top). Scale bar, 50 μm.
Taken together, our results demonstrate that the presence of Sox4b is required for gata2a expression in the pituitary, which itself is required for thyrotrope differentiation.
Sox4b and gata2a are both involved in the expression of gonadotropins (fshβ and lhβ) in the zebrafish developing pituitary
Gata2 is known in mouse to be required for expression of gonadotropins, whereas our own results show that both Gata2a and Sox4b control expression at 48 hpf of gsuα, the hormone subunit common to thyrotropes and gonadotropes. Consequently, we considered the possibility that both transcription factors might play a role also in gonadotropin expression.
Previous studies suggested that fshβ and lhβ transcript levels in the larval zebrafish pituitary are extremely low and detectable only via RT-PCR at 72 hpf (8). Therefore, we first tested the gonadotropin β-subunit mRNA levels by qRT-PCR in RNA extracted from 4-dpf larvae previously microinjected with control MO or MO directed against sox4b or gata2a. The results show that in sox4b morphants (Fig. 9A) and in gata2a morphants (Fig. 9B), both lhβ and fshβ mRNA levels are clearly decreased relative to control MO-injected larvae. When we analyzed lhβ expression by fluorescent in situ hybridization in 4-dpf larvae, we could clearly detect several lhβ-expressing cells in the controls, whereas no or only weakly labeled cells were detected in sox4b morphants (Fig. 9C). As an internal control for the efficiency of in situ hybridization and detection, we used in parallel a probe for the pancreatic marker insulin (ins), whose signal was not affected by sox4bMO injection.
Fig. 9.
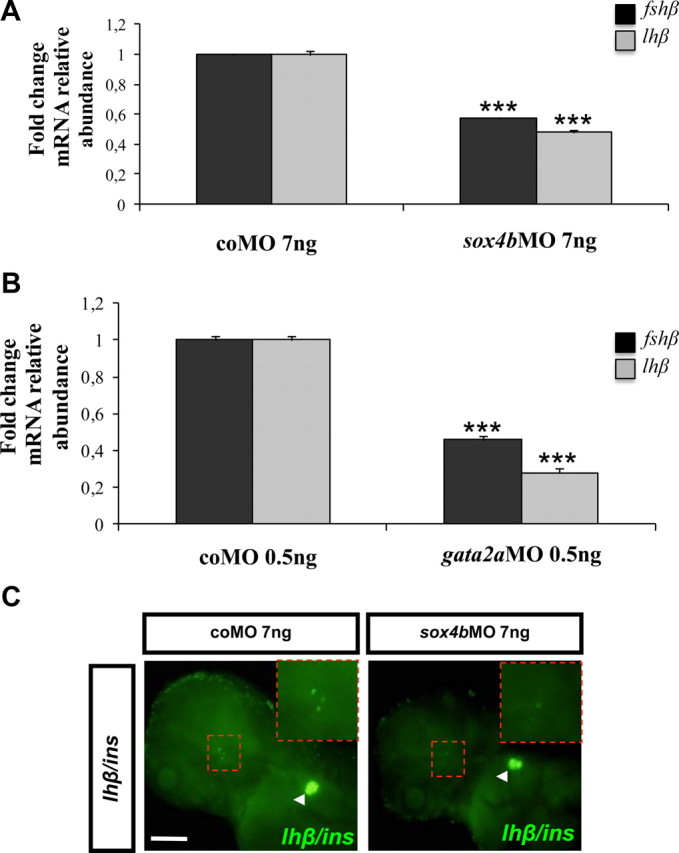
Both Sox4b and Gata2a are required for gonadotropin expression. Expression of lhβ and fshβ was measured by qRT-PCR in RNA extracted from sox4b morphant (A) and gata2a morphant (B) larvae of 4 dpf. The data were normalized relative to the ef1α transcript, and the levels in coMO-injected embryos were arbitrarily set to 1. Mean fold change relative to control morphants is shown with the corresponding sd (n = 3). ***, Significant at P < 0,001. The results shown are representative of three independent experiments. C, Analysis by double-fluorescent WISH for expression of lhβ and ins at 4 dpf previously microinjected with coMO or sox4bMO; pancreatic ins (arrowhead) expression was assessed as invariant control. C, Ventral view (anterior to the left). Scale bar, 200 μm.
Taken together, these results show that Sox4b and Gata2a are both required for gonadotropin expression in zebrafish pituitary.
Discussion
The Sox4 transcription factor is highly conserved in vertebrates, encoded by a single exon, and expressed in different tissues during embryonic and adult life. In mammals, Sox4 is essential for heart, lymphocyte, and thymocyte development (44). Furthermore, Sox4 was shown to be required for normal endocrine pancreas development in mouse (32). Recently, SOX4 expression was found in the human fetal pituitary (33).
In zebrafish, two homologs for the mammalian Sox4 gene exist, only one of them, sox4b, was shown to be required for glucagon-producing cell differentiation (31). In our study, we determined the expression and functional role of Sox4b in the specification of endocrine cell types during anterior pituitary development in zebrafish.
We observed sox4b-expressing cells in a domain located in the pituitary placode at early stages (24 hpf) and in the directly adjacent ventral diencephalon. At 30 hpf, sox4b expression covers most of the lim3-expressing domain (Fig. 1), confirming that sox4b is expressed in pituitary precursor cells. At later stages (48 hpf), sox4b mRNA is detected in the entire forebrain, and a clear signal is detected in the pituitary region expressing tshβ (Fig. 1), gsuα, and the transcription factor gata2a. All these observations confirm that Sox4b is expressed in pituitary cells in zebrafish, consistent with its recently described expression in human fetal pituitary (33).
Loss-of-function studies using MO knockdown or expression of a dominant-negative Sox4 mutant allowed us to uncover a key role for Sox4b in pituitary cell fate specification. Loss of Sox4b function results in dramatic reduction of gsuα, tshβ, lhβ, and fshβ expression and a moderate decrease in gh expression, whereas other pituitary markers are not significantly affected at 48 hpf, indicating that sox4b expression is mainly required for differentiation of the specific gsuα-expressing lineage giving rise to thyrotrope and gonadotrope cells during pituitary development.
Sox4b does not affect expression of the pituitary transcription factors Pit1 and Lim3; however, it is required for expression of the zinc finger transcription factor Gata2a specifically in the zebrafish developing pituitary. The function of Gata2 was intensely studied in the hematopoietic tissues for proliferation and survival of hematopoietic stem cells (45). A function in urogenital development (46) and in adipocyte (47) and endothelial cell (48) differentiation was also described. In mouse, Gata2 expression was shown in thyrotropes and gonadotropes starting at embryonic day 10.5 (12) and transfection studies in cell cultures revealed that Gata2 activates the Tshβ promoter through a synergistic action with Pit1 in thyrotropes (49). Gata factors also stimulate the Gsuα promotor in mouse gonadotrope cells (50). Recently, a mouse Cre-Lox knockout (KO) model was generated where Gata2 gene disruption specifically in Gsuα-expressing cells decreased the number of thyrotropes and gonadotropes at birth and severely reduced Tsh, Lh, and Fsh expression in the adult (43).
In zebrafish, gata2a transcripts are detected in the ventral ectoderm at 75% epiboly and in the yolk syncytial layer at 90% epiboly (39); later, it is expressed in hematopoietic tissues and the central nervous system (51). Similar to mouse, Gata2a can modify the expression of specific genes in erythrocytes (37). In the developing pituitary, we found gata2a expression to be highly overlapping with that of sox4b and gsuα, indicating a close relationship between gata2a and the gsuα cell lineage. We also found that gata2a is expressed in all tshβ-expressing cells at 48 hpf. When we investigated hormone expression in gata2a morphants, we indeed observed a drastic decrease of tshβ and gsuα expression at 48 hpf and of gonadotropins at 4 dpf. These results are consistent with those previously described in the conditional KO mouse model (43). However, the defects observed in zebrafish appear to be much stronger than those reported in mouse; the number of thyrotrope and gonadotrope cells was lower at birth but recovered in the adult, whereas Tsh, Lh, and Fsh expression remained severely reduced in the adult. Thus, in the mouse pituitary, Gata2 appears to be dispensable for initial gonadotrope and thyrotrope cell fate determination but important for expansion of the cell lineage during embryogenesis and for optimal gonadotrope and thyrotrope function in the adult. Unfortunately, no data are available concerning Tshβ, Lhβ, and Fshβ expression at early stages of the conditional KO mouse pituitary development; thus, it remains unclear whether the Gata2 depletion in mouse leads to a drastic delay of thyrotrope and gonadotrope differentiation or whether the recovery in cell number results from a compensatory mechanism, such as the reported up-regulation of Gata3 expression in Gata2-deficient mice (43).
Interestingly, the sox4b and gata2a genes in zebrafish display similar characteristics; both are coexpressed in the same pituitary precursor cells also expressing gsuα, and both are involved in thyrotrope and gonadotrope differentiation. We further showed that sox4b knockdown led to decreased expression of gata2a in the pituitary, suggesting that Sox4b activates gata2a expression. We confirmed this hypothesis by demonstrating that exogenous Gata2a expression can compensate for the lack of Sox4b function in tshβ and gsuα cell differentiation in sox4b morphants. Our in situ experiments show that sox4b is expressed at later stages (48 hpf) in the hypothalamus of the developing zebrafish; thus, an indirect effect of hypothalamic signaling on pituitary gata2 expression can be envisaged. However, although we cannot completely rule out a role of hypothalamic Sox4b, the perfect coexpression of sox4b and gata2a in gsuα-expressing cells argues in favor of a cell-autonomous regulation of gata2a expression by the Sox4b transcription factor.
We show that the expression of the pan-pituitary marker lim3 is unchanged in sox4b knockdown embryos, indicating that the number of pituitary-specified cells remains unaltered, whereas their capacity to terminally differentiate is affected. Furthermore, thyrotrope cells depend on the transcription factor Pit1 for their differentiation. Our results clearly show that pit1 expression is not affected in sox4b morphants and, conversely, that sox4b and gata2a expression is not affected in pit1 mutants. Thus, the sox4b/gata2a cascade acts independently of Pit1 to promote gsuα and tshβ expression in the pit1-expressing thyrotropes, consistent with the synergistic activation of the Tshβ promoter that was described in mouse (49).
Another cell lineage that is affected in the sox4b morphants is the somatotropes expressing gh. Although less affected than the thyrotrope and gonadotrope cells, a clear decrease in gh expression was observed in the sox4b morphants in zebrafish. In the conditional Gata2 KO mouse model, Gh expression was normal at birth, whereas a transient growth retardation was observed in males between wk 3 and 9 (43). In this model, Gata2 gene depletion is obtained by expressing the CRE recombinase in Gsuα-expressing cells; thus, the somatotrope cells are presumably Gata2 positive. However, no Gata2 expression was observed in mouse somatotropes, and we show also here no colocalization of gata2a and gh mRNA in zebrafish, indicating that the effect of Sox4b or Gata2a depletion on gh expression might be cell nonautonomous, through altering the numbers or properties of other cell types. The precise significance and mechanism of the Sox4b/Gata2a regulatory cascade on GH expression will require further investigation.
Numerous reports have been published on Fsh and Lh in teleosts. Both hormones are synthesized in gonadotrope cells in two separate populations exhibiting distinct patterns of distribution in the zebrafish pituitary (52). Their expression is low in zebrafish larvae and was only detectable by RT-PCR at 72 hpf (8), whereas sexual differentiation of the gonads was shown to start in 3-wk-old juveniles (53). Using the more sensitive fluorescent in situ hybridization, we were able to observe several lhβ-positive cells at 72 hpf in control embryos and observed a clear decrease in the number of these cells in sox4b morphants. qRT-PCR analysis further confirmed the decreased expression of both lhβ and fshβ, consistent with previous studies in mammalian cell culture revealing the activation of the gonadotropin subunit genes Gsuα (50) and Lhβ (54) by Gata factors. Our results clearly show that the Sox4b and Gata2a factors are required for formation of lhβ- and fshβ-expressing cells in developing zebrafish; thus, they represent to date the only factors specifically involved in gonadotrope cell differentiation in zebrafish.
Recently, induction of SOX4 expression by TGFβ1 was shown in the human HP75 cell line derived from a silent gonadotropinoma (55), suggesting that Sox4 genes could be targets for TGFβ/bone morphogenetic protein (BMP) signaling pathways. The TGFβ family member activin is known to activate gonadotropin expression in the adult pituitary, whereas inhibin represses this effect (56). A function for activin in maintaining cellular homeostasis within the pituitary was also proposed. During pituitary development in the mouse, a crucial role was demonstrated for BMP, also belonging to the TGFβ family. BMP4 is required early for formation of the Rathke's pouch and thus for development of the entire pituitary, whereas BMP2 exerts a ventralizing effect within the developing Rathke's pouch, thus favoring the formation of, e.g. gonadotropes (57). These observations are consistent with a role for Sox4b in the transduction of TGFβ signaling at least in gonadotropes; however, in zebrafish, manipulation of BMP signaling failed to cause any defect in pituitary development (58). Thus, at present, the relationship between Sox4 factors and TGFβ signaling remains an open question.
Taken together, our results show that the Sry-related HMG-box transcription factor Sox4b is involved in the differentiation of thyrotrope and gonadotrope cells and that this function is mediated by specifically activating gata2a expression in gsuα-expressing pituitary precursor cells.
Acknowledgments
We thank the GIGA-R zebrafish facility for providing zebrafish adults and fertilized eggs and the GIGA-R Transcriptomics platform for DNA sequencing and RNA quality control.
This work was supported by the Fonds National pour la Recherche Scientifique (FNRS) 2.4542.00/2.4555.99/2.4561.10/2.4631.11/2.4531.09, the “Belgian Science Policy” agency: Pôle d'attraction Interuniversitaire P5/35, the University of Liège: “GAME” project to M.M. and the Special Fund for Research to P.M. M.L. held a fellowship from the Walloon Region and the “Belgian Science Policy” agency. Y.Q. was supported by the European Commission and the Walloon Region (Alma-in-Silico project). M.M. is a Chercheur Qualifié du FNRS.
Present address for M.L.: Institute of Hematology, Faculty of Medecine, Universidad Austral, Valdivia, Chile.
Present address for A.M.: Department of Medicine, University of California, San Francisco, Diabetes Center, Hormone Research Institute, 513 Parnassus Avenue, San Francisco, California 94143.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMP
- Bone morphogenetic protein
- coMO
- control morpholino
- dpf
- days after fertilization
- Gsuα
- glycoprotein α-subunit
- hpf
- hours after fertilization
- KO
- knockout
- MO
- morpholino
- PPE
- preplacodal ectoderm
- qRT-PCR
- quantitative real-time RT-PCR
- Prl
- prolactin
- SOX
- SRY-related HMG box
- WISH
- whole-mount in situ hybridization
- wt
- wild type.
References
- 1. Mehta A , Dattani MT. 2008. Developmental disorders of the hypothalamus and pituitary gland associated with congenital hypopituitarism. Best Pract Res Clin Endocrinol Metab 22:191–206 [DOI] [PubMed] [Google Scholar]
- 2. Zhu X , Lin CR , Prefontaine GG , Tollkuhn J , Rosenfeld MG. 2005. Genetic control of pituitary development and hypopituitarism. Curr Opin Genet Dev 15:332–340 [DOI] [PubMed] [Google Scholar]
- 3. Kelberman D , Dattani MT. 2007. Hypothalamic and pituitary development: novel insights into the aetiology. Eur J Endocrinol 157(Suppl 1):S3–S14 [DOI] [PubMed] [Google Scholar]
- 4. Rhodes SJ , DiMattia GE , Rosenfeld MG. 1994. Transcriptional mechanisms in anterior pituitary cell differentiation. Curr Opin Genet Dev 4:709–717 [DOI] [PubMed] [Google Scholar]
- 5. Zhu Y , Stiller JW , Shaner MP , Baldini A , Scemama JL , Capehart AA. 2004. Cloning of somatolactin alpha and beta cDNAs in zebrafish and phylogenetic analysis of two distinct somatolactin subtypes in fish. J Endocrinol 182:509–518 [DOI] [PubMed] [Google Scholar]
- 6. Lopez M , Nica G , Motte P , Martial JA , Hammerschmidt M , Muller M. 2006. Expression of the somatolactin β gene during zebrafish embryonic development. Gene Expr Patterns 6:156–161 [DOI] [PubMed] [Google Scholar]
- 7. Dutta S , Dietrich JE , Aspöck G , Burdine RD , Schier A , Westerfield M , Varga ZM. 2005. pitx3 defines an equivalence domain for lens and anterior pituitary placode. Development 132:1579–1590 [DOI] [PubMed] [Google Scholar]
- 8. Nica G , Herzog W , Sonntag C , Nowak M , Schwarz H , Zapata AG , Hammerschmidt M. 2006. Eya1 is required for lineage-specific differentiation, but not for cell survival in the zebrafish adenohypophysis. Dev Biol 292:189–204 [DOI] [PubMed] [Google Scholar]
- 9. Zilinski CA , Shah R , Lane ME , Jamrich M. 2005. Modulation of zebrafish pitx3 expression in the primordia of the pituitary, lens, olfactory epithelium and cranial ganglia by hedgehog and nodal signaling. Genesis 41:33–40 [DOI] [PubMed] [Google Scholar]
- 10. Li S , Crenshaw EB , Rawson EJ , Simmons DM , Swanson LW , Rosenfeld MG. 1990. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature 347:528–533 [DOI] [PubMed] [Google Scholar]
- 11. Nica G , Herzog W , Sonntag C , Hammerschmidt M. 2004. Zebrafish pit1 mutants lack three pituitary cell types and develop severe dwarfism. Mol Endocrinol 18:1196–1209 [DOI] [PubMed] [Google Scholar]
- 12. Dasen JS , O'Connell SM , Flynn SE , Treier M , Gleiberman AS , Szeto DP , Hooshmand F , Aggarwal AK , Rosenfeld MG. 1999. Reciprocal interactions of Pit1 and GATA2 mediate signaling gradient-induced determination of pituitary cell types. Cell 97:587–598 [DOI] [PubMed] [Google Scholar]
- 13. Wegner M. 1999. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res 27:1409–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Avilion AA , Nicolis SK , Pevny LH , Perez L , Vivian N , Lovell-Badge R. 2003. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev 17:126–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kondoh H , Uchikawa M , Kamachi Y. 2004. Interplay of Pax6 and SOX2 in lens development as a paradigm of genetic switch mechanisms for cell differentiation. Int J Dev Biol 48:819–827 [DOI] [PubMed] [Google Scholar]
- 16. Kamachi Y , Uchikawa M , Collignon J , Lovell-Badge R , Kondoh H. 1998. Involvement of Sox1, 2 and 3 in the early and subsequent molecular events of lens induction. Development 125:2521–2532 [DOI] [PubMed] [Google Scholar]
- 17. Lang RA. 2004. Pathways regulating lens induction in the mouse. Int J Dev Biol 48:783–791 [DOI] [PubMed] [Google Scholar]
- 18. Ikeda T , Kawaguchi H , Kamekura S , Ogata N , Mori Y , Nakamura K , Ikegawa S , Chung UI. 2005. Distinct roles of Sox5, Sox6, and Sox9 in different stages of chondrogenic differentiation. J Bone Miner Metab 23:337–340 [DOI] [PubMed] [Google Scholar]
- 19. Smits P , Dy P , Mitra S , Lefebvre V. 2004. Sox5 and Sox6 are needed to develop and maintain source, columnar, and hypertrophic chondrocytes in the cartilage growth plate. J Cell Biol 164:747–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pevny L , Placzek M. 2005. SOX genes and neural progenitor identity. Curr Opin Neurobiol 15:7–13 [DOI] [PubMed] [Google Scholar]
- 21. Solomon NM , Ross SA , Morgan T , Belsky JL , Hol FA , Karnes PS , Hopwood NJ , Myers SE , Tan AS , Warne GL , Forrest SM , Thomas PQ. 2004. Array comparative genomic hybridisation analysis of boys with X linked hypopituitarism identifies a 3.9 Mb duplicated critical region at Xq27 containing SOX3. J Med Genet 41:669–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Woods KS , Cundall M , Turton J , Rizotti K , Mehta A , Palmer R , Wong J , Chong WK , Al-Zyoud M , El-Ali M , Otonkoski T , Martinez-Barbera JP , Thomas PQ , Robinson IC , Lovell-Badge R , Woodward KJ , Dattani MT. 2005. Over- and underdosage of SOX3 is associated with infundibular hypoplasia and hypopituitarism. Am J Hum Genet 76:833–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laumonnier F , Ronce N , Hamel BC , Thomas P , Lespinasse J , Raynaud M , Paringaux C , Van Bokhoven H , Kalscheuer V , Fryns JP , Chelly J , Moraine C , Briault S. 2002. Transcription factor SOX3 is involved in X-linked mental retardation with growth hormone deficiency. Am J Hum Genet 71:1450–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kelberman D , de Castro SC , Huang S , Crolla JA , Palmer R , Gregory JW , Taylor D , Cavallo L , Faienza MF , Fischetto R , Achermann JC , Martinez-Barbera JP , Rizzoti K , Lovell-Badge R , Robinson IC , Gerrelli D , Dattani MT. 2008. SOX2 plays a critical role in the pituitary, forebrain, and eye during human embryonic development. J Clin Endocrinol Metab 93:1865–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kelberman D , Rizzoti K , Avilion A , Bitner-Glindzicz M , Cianfarani S , Collins J , Chong WK , Kirk JM , Achermann JC , Ross R , Carmignac D , Lovell-Badge R , Robinson IC , Dattani MT. 2006. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J Clin Invest 116:2442–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Busslinger M. 2004. Transcriptional control of early B cell development. Annu Rev Immunol 22:55–79 [DOI] [PubMed] [Google Scholar]
- 27. Ya J , Schilham MW , de Boer PA , Moorman AF , Clevers H , Lamers WH. 1998. Sox4-deficiency syndrome in mice is an animal model for common trunk. Circ Res 83:986–994 [DOI] [PubMed] [Google Scholar]
- 28. Cheung M , Abu-Elmagd M , Clevers H , Scotting PJ. 2000. Roles of Sox4 in central nervous system development. Brain Res Mol Brain Res 79:180–191 [DOI] [PubMed] [Google Scholar]
- 29. Hunt SM , Clarke CL. 1999. Expression and hormonal regulation of the Sox4 gene in mouse female reproductive tissues. Biol Reprod 61:476–481 [DOI] [PubMed] [Google Scholar]
- 30. Friedman RS , Bangur CS , Zasloff EJ , Fan L , Wang T , Watanabe Y , Kalos M. 2004. Molecular and immunological evaluation of the transcription factor SOX-4 as a lung tumor vaccine antigen. J Immunol 172:3319–3327 [DOI] [PubMed] [Google Scholar]
- 31. Mavropoulos A , Devos N , Biemar F , Zecchin E , Argenton F , Edlund H , Motte P , Martial JA , Peers B. 2005. sox4b is a key player of pancreatic α-cell differentiation in zebrafish. Dev Biol 285:211–223 [DOI] [PubMed] [Google Scholar]
- 32. Wilson ME , Yang KY , Kalousova A , Lau J , Kosaka Y , Lynn FC , Wang J , Mrejen C , Episkopou V , Clevers HC , German MS. 2005. The HMG box transcription factor Sox4 contributes to the development of the endocrine pancreas. Diabetes 54:3402–3409 [DOI] [PubMed] [Google Scholar]
- 33. Ma Y , Qi X , Du J , Song S , Feng D , Qi J , Zhu Z , Zhang X , Xiao H , Han Z , Hao X. 2009. Identification of candidate genes for human pituitary development by EST analysis. BMC Genomics 10:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Westerfield M. 1995. The zebrafish book: guide for the laboratory use of zebrafish (Danio rerio). 3rd ed Eugene, OR: University of Oregon Press [Google Scholar]
- 35. Kimmel CB , Ballard WW , Kimmel SR , Ullmann B , Schilling TF. 1995. Stages of embryonic development of the zebrafish. Dev Dyn 203:253–310 [DOI] [PubMed] [Google Scholar]
- 36. Herzog W , Sonntag C , Walderich B , Odenthal J , Maischein HM , Hammerschmidt M. 2004. Genetic analysis of adenohypophysis formation in zebrafish. Mol Endocrinol 18:1185–1195 [DOI] [PubMed] [Google Scholar]
- 37. Galloway JL , Wingert RA , Thisse C , Thisse B , Zon LI. 2005. Loss of gata1 but not gata2 converts erythropoiesis to myelopoiesis in zebrafish embryos. Dev Cell 8:109–116 [DOI] [PubMed] [Google Scholar]
- 38. Hauptmann G , Gerster T. 1994. Two-color whole-mount in situ hybridization to vertebrate and Drosophila embryos. Trends Genet 10:266. [DOI] [PubMed] [Google Scholar]
- 39. Detrich HW , Kieran MW , Chan FY , Barone LM , Yee K , Rundstadler JA , Pratt S , Ransom D , Zon LI. 1995. Intraembryonic hematopoietic cell migration during vertebrate development. Proc Natl Acad Sci USA 92:10713–10717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Glasgow E , Karavanov AA , Dawid IB. 1997. Neuronal and neuroendocrine expression of lim3, a LIM class homeobox gene, is altered in mutant zebrafish with axial signaling defects. Dev Biol 192:405–419 [DOI] [PubMed] [Google Scholar]
- 41. Herzog W , Zeng X , Lele Z , Sonntag C , Ting JW , Chang CY , Hammerschmidt M. 2003. Adenohypophysis formation in the zebrafish and its dependence on sonic hedgehog. Dev Biol 254:36–49 [DOI] [PubMed] [Google Scholar]
- 42. Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Charles MA , Saunders TL , Wood WM , Owens K , Parlow AF , Camper SA , Ridgway EC , Gordon DF. 2006. Pituitary-specific Gata2 knockout: effects on gonadotrope and thyrotrope function. Mol Endocrinol 20:1366–1377 [DOI] [PubMed] [Google Scholar]
- 44. Schilham MW , Oosterwegel MA , Moerer P , Ya J , de Boer PA , van de Wetering M , Verbeek S , Lamers WH , Kruisbeek AM , Cumano A , Clevers H. 1996. Defects in cardiac outflow tract formation and pro-B-lymphocyte expansion in mice lacking Sox-4. Nature 380:711–714 [DOI] [PubMed] [Google Scholar]
- 45. Shimizu R , Yamamoto M. 2005. Gene expression regulation and domain function of hematopoietic GATA factors. Semin Cell Dev Biol 16:129–136 [DOI] [PubMed] [Google Scholar]
- 46. Zhou Y , Lim KC , Onodera K , Takahashi S , Ohta J , Minegishi N , Tsai FY , Orkin SH , Yamamoto M , Engel JD. 1998. Rescue of the embryonic lethal hematopoietic defect reveals a critical role for GATA-2 in urogenital development. Embo J 17:6689–6700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tong Q , Tsai J , Tan G , Dalgin G , Hotamisligil GS. 2005. Interaction between GATA and the C/EBP family of transcription factors is critical in GATA-mediated suppression of adipocyte differentiation. Mol Cell Biol 25:706–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Minami T , Murakami T , Horiuchi K , Miura M , Noguchi T , Miyazaki J , Hamakubo T , Aird WC , Kodama T. 2004. Interaction between hex and GATA transcription factors in vascular endothelial cells inhibits flk-1/KDR-mediated vascular endothelial growth factor signaling. J Biol Chem 279:20626–20635 [DOI] [PubMed] [Google Scholar]
- 49. Gordon DF , Lewis SR , Haugen BR , James RA , McDermott MT , Wood WM , Ridgway EC. 1997. Pit-1 and GATA-2 interact and functionally cooperate to activate the thyrotropin β-subunit promoter. J Biol Chem 272:24339–24347 [DOI] [PubMed] [Google Scholar]
- 50. Steger DJ , Hecht JH , Mellon PL. 1994. GATA-binding proteins regulate the human gonadotropin α-subunit gene in the placenta and pituitary gland. Mol Cell Biol 14:5592–5602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Meng A , Tang H , Ong BA , Farrell MJ , Lin S. 1997. Promoter analysis in living zebrafish embryos identifies a cis-acting motif required for neuronal expression of GATA-2. Proc Natl Acad Sci USA 94:6267–6272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. So WK , Kwok HF , Ge W. 2005. Zebrafish gonadotropins and their receptors. II. Cloning and characterization of zebrafish follicle-stimulating hormone and luteinizing hormone subunits: their spatial-temporal expression patterns and receptor specificity. Biol Reprod 72:1382–1396 [DOI] [PubMed] [Google Scholar]
- 53. Uchida D , Yamashita M , Kitano T , Iguchi T. 2002. Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J Exp Biol 205:711–718 [DOI] [PubMed] [Google Scholar]
- 54. Lo A , Zheng W , Gong Y , Crochet JR , Halvorson LM. 2011. GATA transcription factors regulate LHβ gene expression. J Mol Endocrinol 47:45–58 [DOI] [PubMed] [Google Scholar]
- 55. Ruebel KH , Leontovich AA , Tanizaki Y , Jin L , Stilling GA , Zhang S , Coonse K , Scheithauer BW , Lombardero M , Kovacs K , Lloyd RV. 2008. Effects of TGFβ1 on gene expression in the HP75 human pituitary tumor cell line identified by gene expression profiling. Endocrine 33:62–76 [DOI] [PubMed] [Google Scholar]
- 56. Bilezikjian LM , Justice NJ , Blackler AN , Wiater E , Vale WW. 4 February 2012. Cell-type specific modulation of pituitary cells by activin, inhibin and follistatin. Mol Cell Endocrinol 10.1016/j.mce.2012.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhu X , Gleiberman AS , Rosenfeld MG. 2007. Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev 87:933–963 [DOI] [PubMed] [Google Scholar]
- 58. Pogoda HM , Hammerschmidt M. 2009. How to make a teleost adenohypophysis: molecular pathways of pituitary development in zebrafish. Mol Cell Endocrinol 312:2–13 [DOI] [PubMed] [Google Scholar]