Fig. 1.
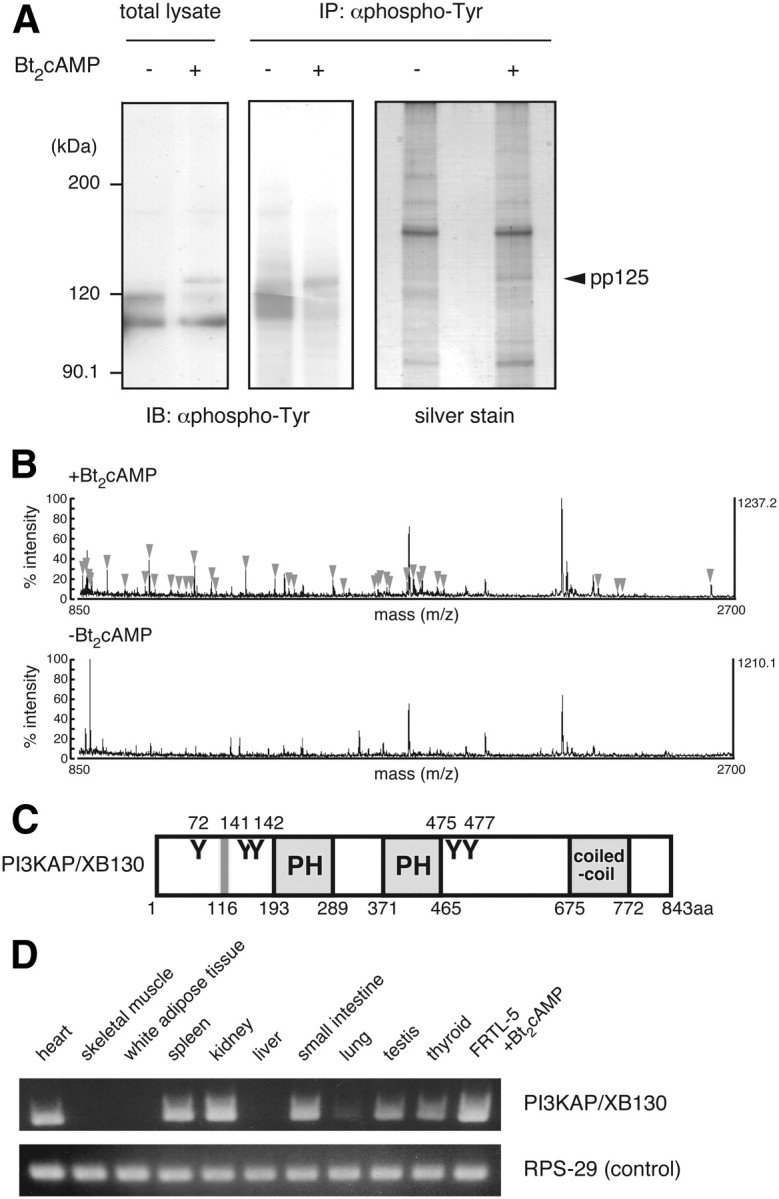
Identification of the 125-kDa tyrosine-phosphorylated protein (p125). A, Quiescent FRTL-5 cells were treated with 1 mm Bt2cAMP for 24 h. Cell lysates were prepared and subjected to immunoprecipitation with anti-phosphotyrosine (αphospho-Tyr) antibody, as described in Materials and Methods. Samples were analyzed by immunoblotting with anti-phosphotyrosine antibody or silver staining. B, The band corresponding to p125 in the cAMP-treated sample (indicated as pp125) was subjected to MALDI-TOF MS analysis. The gel region with a similar electrophoretic mobility in the untreated sample was used as a negative control. MS peaks detected only in the Bt2cAMP-treated sample (arrowheads) were analyzed by peptide mass finger printing. C, Schematic diagram of mouse PI3KAP/XB130. Y represents putative tyrosine phosphorylation sites for Src family kinases, PH is the PH domain, and coiled-coil is the coiled-coil domain. Gray line represents the SH3 domain-binding motif. Amino acid numbers of putative tyrosine phosphorylation sites, and ends of each domain/motif are indicated. D, PI3KAP/XB130 mRNA levels in rat tissues or FRTL-5 cells treated with 1 mm Bt2cAMP for 24 h were analyzed by RT-PCR. RPS-29 was analyzed as a loading control.
