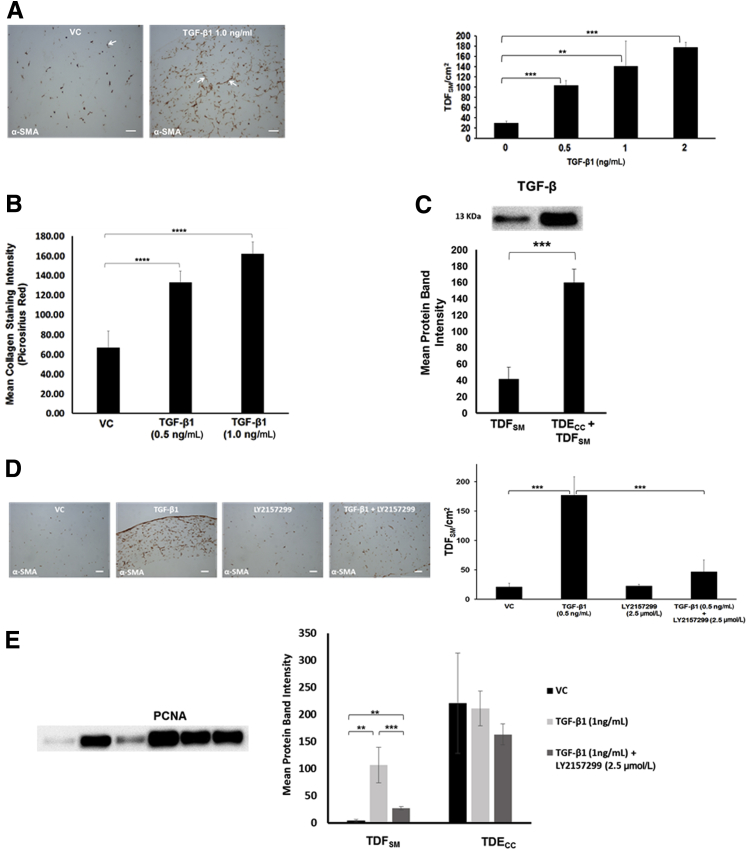
Figure 10.
A: Recombinant human TGF-β1 (R&D Systems, Inc., Minneapolis, MN) induces significant concentration-dependent increases in α-SMA–positive fibroblastic cell number/cm2 gel section area when exogenously added to 3D monocultures of TDFSM cells. Initial viable cell plating density of TDFSM cells = 8 × 105/gel. TGF-β1 at the indicated concentration levels was added daily over a 72-hour period beginning at 4 hours after initial cell plating to cultures maintained in standard medium containing 1.0% FBS. Arrows point to representative α-SMA–positive TDFSM myofibroblastic cells. B: Corresponding concentration-dependent effect in 3D mono-cell gel cultures of TDFSM cells of exogenously added TGF-β1 on significantly enhancing dense fibrous collagen production measured as picrosirius red staining intensity under polarized light over that produced in vehicle control (VC) cultures. C: Comparative quantitative Western blot analysis of mature TGF-β protein expression in 3D gel cultures of TDFSM myofibroblastic cells alone and in co-culture with TDECC cholangiocarcinoma cells. Initial viable plating densities for TDFSM cells = 1.6 × 106 cells per gel; for TDECC cells = 4 × 105 cells per gel. Western blotting was performed on total gel protein lysates prepared from cultures that were maintained in serum-free medium for 48 hours, beginning 1 day after initial cell plating. Primary antibody: rabbit polyclonal TGF-β (ab66043, Abcam Inc.) at 1:500 followed by secondary antibody conjugated with horseradish peroxidase (1:3000). D: Concomitantly added LY2157299 significantly blocks the TGF-β1–induced increase in TDFSM cells/cm2 area of histological sections prepared within the 3D collagen gels. E: Effect of exogenously added recombinant human TGF-β1 alone versus in the presence of LY2157299 on PCNA protein expression in 3D gel monocultures of TDFSM cells compared with those of TDECC cells. Initial viable plating densities were 1.6 × 106 TDFSM cells/gel and 4 × 105 TDECC cells/gel, respectively. Cultures were treated at the indicated concentrations with either recombinant human TGF-β1 alone, TGF-β1 + LY2157299, or VC, each of which was added daily in the presence of standard medium containing 1.0% FBS beginning at 4 hours after initial cell plating and then continued daily at 24, 48, and 72 hours after the start of treatment. The cultures were then processed for Western blotting at 96 hours after the initial cell plating. Data are expressed as means ± SD from measurements made on 3 separate cultures per data point (A–E). ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001. Scale bars = 100 μm (A and D).