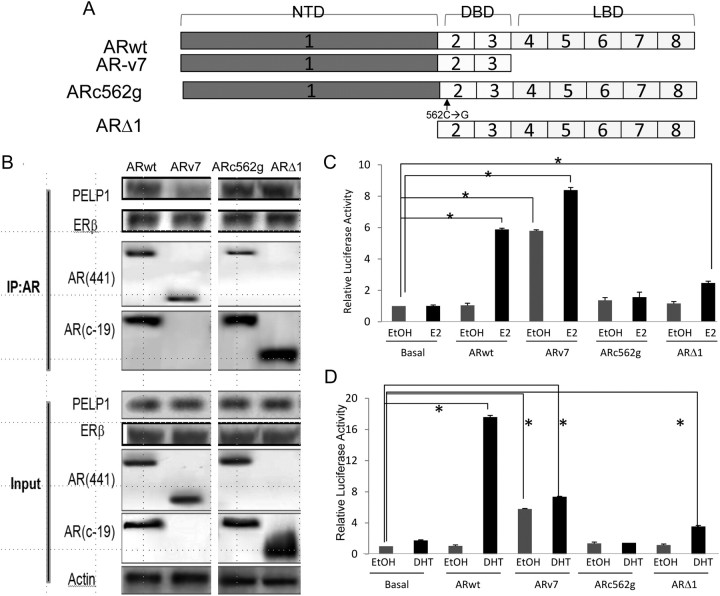
Fig. 5.
Effect of AR mutants on AR-PELP1 binding and E2-mediated transactivation. A, Structures of five AR constructs used: ARwt is the full-length AR, ARv7 is an AR splice variant that lacks the C-terminal ligand-binding domain, ARc562g is a point mutant AR construct that does not translocate to the nucleus upon DHT treatment, and ARΔ1 is a synthetic construct lacking AR amino terminus and the transactivation domain. NTD, Amino-terminal domain; DBD, DNA-binding domain; LBD, ligand-binding domain. B, PC-3 cells were transiently transfected with these AR constructs and then evaluated for complex formation between AR and PELP1 and AR and ERβ by immunoprecipitation with the AR441 antibody (directed against an epitope on the amino terminus of AR) or the AR c-19 antibody (directed against an epitope on the carboxy terminus of AR). Immunoprecipitation of extracts from PC3 cells transfected with ARwt, ARv7, and ARc562g was performed with the AR 441 antibody (against the N terminus), whereas those from cells transfected with ARΔ1 were perfomed with the AR c-19 antibody (against the C terminus). The immunoblotting was performed with antibodies to PELP1 and ERβ and both the AR-441 antibody and the AR c-19 antibody. Input lanes reveal the expression and immunoreactivity of PELP1, ERβ, and AR in these transiently PC-3-transfected cells (bottom four rows). As expected, both the ARwt and ARc562g are recognized by both AR antibodies. The ARΔ1 splice variant is recognized by only by the C-terminal antibody, whereas the ARv7 splice variant is only recognized by the N-terminal antibody. Transcription in PC-3 cells transiently transfected with AR splice variants, along with an ARE luciferase promoter, was evaluated in the absence and presence of E2 (10 nm) (C) or DHT (10 nm) for 24 h (D). IP, Immunoprecipitation; *, statistically significant.