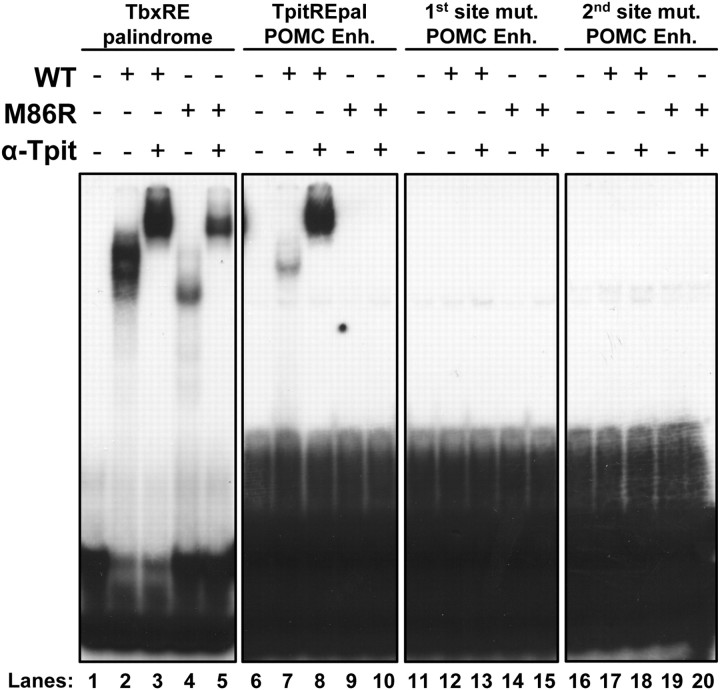
Fig. 5.
Tpit dimers bind the TpitREpal in vitro. A, Gel retardation was used to assess binding of either wild-type (WT) Tpit or mutant M86R to the consensus TbxRE palindrome and to TpitREpal probes, or to probes mutated for each half-site of the TpitREpal, as indicated. Equivalent amounts of 32P-labeled probes were used. The first panel is an overnight film exposure, whereas the last three panels are 3 d exposures of the same gel. Tpit binding to the consensus TbxRE probe is stronger than the TpitREpal (compare lanes 2 and 7), but Tpit:DNA complexes are supershifted by Tpit antibody in both cases (lanes 3 and 8). The M86R Tpit dimerization-mutant exhibit monomeric binding on the consensus TbxRE, contrasting with an absence of TpitREpal probe retardation (lanes 4 and 9). Mutant probes for either half-site of the TpitREpal do not exhibit any binding (lanes 11–20).