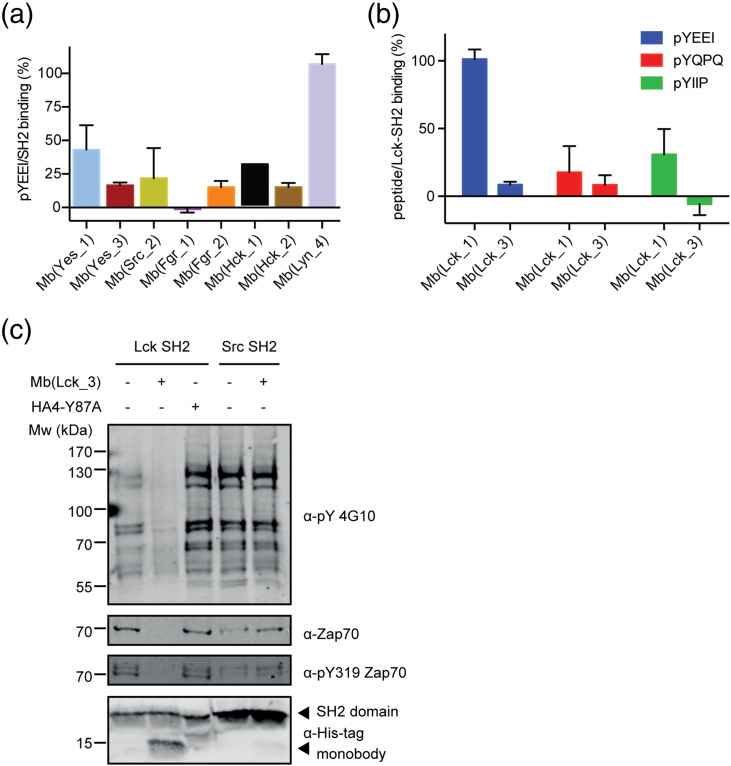
Fig. 4.
Inhibition of pY peptide/SH2 interaction by monobodies. (a) The graph shows relative pYEEI peptide binding (in %) to SH2 domain in the presence of a monobody. All eight SH2 domains have been measured without and in presence of the monobody selected for the respective on-target. The pYEEI peptide in isolation and the SH2/pYEEI complex were set to 0% and 100% binding, respectively. Accordingly, the reduction in binding observed in conjunction with a monobody is expressed as a percentage. Each data point corresponds to the average of at least two repeats +/− SD. (b) Relative peptide binding (in %) of three different peptides (pYEEI, pYQPQ, pYIIP) to the Lck SH2 domain in the presence of each of the two Lck monobodies [Mb(Lck_1) or Mb(Lck_3)]. The full sequence of the peptides can be found in the Materials and Methods section. (c) The monobodies Mb(Lck_3) or HA4-Y87A or buffer alone was added to biotinylated Lck or Src SH2 domains immobilized on Streptavidin-coated beads prior to incubation with lysate from stimulated Jurkat T cells. Immunoblot analysis after pull-down using an anti-pY antibody for a 40% fraction of beads (upper blot), using an anti-Zap70 antibody and an anti-phospho-Zap70 (pY319) antibody for a 40% fraction of beads (second and third blot from top), and using an anti-His-tag antibody to detect the recombinant SH2 domains and monobodies for a 10% fraction of beads (lower blot).