Abstract
Background
No randomized controlled trials of screening colonoscopies have been completed, and ongoing trials exclude subjects aged 75 years or older. The Medicare program, however, reimburses screening colonoscopies without an upper age limit.
Objective
To evaluate the effectiveness and safety of screening colonoscopy to prevent colorectal cancer in individuals aged 70–74 and 75–79.
Design Setting
Large-scale, population-based, prospective study. The observational data was used to emulate a target trial with 2 arms: colonoscopy screening and no screening.
Participants
1,355,692 Medicare individuals (2004–2012) aged 70–79 at average risk for CRC who used Medicare preventive services and had no previous diagnostic or surveillance colonoscopies in the previous five years.
Measurements
Eight-year risk of CRC and 30-day risk of adverse events.
Results
In the 70–74 age group, the 8-year risk of CRC (95% CI) was 2.19% (2.00, 2.37) in the colonoscopy screening arm and 2.62% (2.56, 2.67) in the no screening arm; absolute risk difference −0.42% (−0.24, −0.63). In the 75–79 age group, the 8-year risk of CRC (95% CI) was 2.84% (2.54, 3.13) in the colonoscopy screening arm and 2.97% (2.92, 3.03) in the no screening arm; risk difference −0.14% (−0.41, 0.16). The excess 30-day risk of any adverse event in the colonoscopy arm was 5.6 adverse events per 1,000 individuals (95% CI 4.4–6.8) in the 70–74 age group and 10.3 adverse events per 1,000 individuals (95% CI 8.6–11.1) in the 75–79 group.
Limitations
CRC-specific mortality was not available, but we studied CRC incidence and stage at diagnosis.
Conclusions
Our findings suggest a modest benefit of screening colonoscopy for the prevention of CRC in beneficiaries aged 70–74 years, and a smaller benefit in older beneficiaries. The risk of adverse events was low, but greater among older individuals.
Introduction
There are 132,000 new cases of colorectal cancer (CRC) and 50,000 CRC-related deaths each year in the US (1). Colonoscopy is expected to reduce CRC mortality by detecting asymptomatic, curable cancers and CRC incidence by detecting and removing precancerous polyps. However, despite being widely used in the US (2), no randomized controlled trials of screening colonoscopy have been completed. The findings of three ongoing randomized trials (3–5) will be not be available before the mid 2020s. Previously conducted randomized trials showed the effectiveness of other screening methods: periodic fecal occult blood testing (FOBT) reduces CRC mortality (6–10) and sigmoidoscopy (performed once (11–14) or twice within 3–5 years(15)) reduces both CRC incidence and CRC mortality (16).
Colonoscopy is an invasive, resource-demanding procedure that requires a thorough large bowel cleansing and often patient sedation, and that carries a risk of complications such as bowel perforations. Establishing the effectiveness and safety of colonoscopy is important because less burdensome screening methods (FOBT and sigmoidoscopy) are available. Currently, the United States Preventive Service Task Force (USPSTF) recommends routine CRC screening using any screening tests for individuals at average CRC risk, from age 50 up through age 75, and recommends individualizing screening decisions for individuals aged 76–85 years (17). Other guidelines recommend colonoscopy screening without an upper age limit (18,19). None of the ongoing colonoscopy trials include individuals over age 75 (only one includes individuals age 70 or older, eTable 1), but healthy persons over 75 could live sufficiently long to benefit from CRC screening.
Medicare has reimbursed screening colonoscopies for over a decade without an upper age limit. We use the extensive experience of Medicare beneficiaries to estimate the effectiveness of screening colonoscopy for the prevention of CRC among elderly individuals with no recent history of colonoscopy, colorectal cancer, or adenomas. We study individuals aged 70–74 and 75–79 years separately.
Methods
Study data
For a random 20% sample of Medicare beneficiaries from the years 1999 to 2012, we extracted information on demographic characteristics (age, sex, race, original reason for Medicare entitlement, and census bureau division), enrollment characteristics (reason for entitlement, enrollment type and period) and Medicare Chronic Conditions Data Warehouse condition categories (including CRC diagnosis) from the denominator files; colonoscopies and FOBTs from the outpatient standard analytic files, the inpatient hospital claims, and the carrier files (to identify physician services). We used the outpatient and carrier files to extract information on the use of preventive services and wellness visits. We computed a combined comorbidity score (20) and used the procedure codes on the colonoscopy claim and the presence of a pathology bill for examination of a colorectal polyp or biopsy within seven days of the procedure to classify colonoscopies as having performed a polypectomy or not (21). We used the SEER-Medicare linked dataset (22) (years 1999 to 2009) to evaluate CRC stage (see Supplementary material for details).
Eligibility criteria
Our analyses included individuals aged 70–79 without history of prior colorectal cancer who, in the five years before baseline (see below), had no history of adenoma, inflammatory bowel disease or colectomy, had not received a colonoscopy, sigmoidoscopy or FOBT. These eligibility criteria are as similar as possible to those of the ongoing colonoscopy trials (3–5) (eTable 1 in the Supplementary material), which generally target average-risk population.
To ensure complete capture of health information we included only individuals enrolled in Medicare parts A and B, and not in Medicare Advantage, during the preceding five years. To reduce the probability of including individuals who received colonoscopy for reasons other than screening, we (i) excluded those who had received an abdominal CT scan, barium enema or a diagnosis (23) of anemia, gastrointestinal bleeding, constipation, diarrhea, abdominal pain, irritable bowel syndrome, bowel habits change, weight loss, ischemic bowel disease, or diverticular disease in the previous 6 months, and (ii) included only health-conscious individuals who had received at least two out of the three preventive services offered yearly by Medicare for the average population (annual wellness visit, influenza vaccine, breast cancer screening and prostate cancer screening) in the previous two years (24). Though the effectiveness of some of these services is questionable (PSA-based prostate cancer screening), we only used them here as surrogates for health consciousness. We relax these constraints in sensitivity analyses (see Supplementary material).
Treatment arms and follow-up
To emulate a trial of screening colonoscopy and CRC incidence in the elderly, we exploited the experiences of Medicare beneficiaries after their 70th birthdate. Specifically, we identified all 70-year old beneficiaries who met the eligibility criteria on the day they turn 70 (baseline) and followed them until CRC diagnosis, death, violation of Medicare enrolment criteria, or December 2012, whichever occurred earlier. At baseline, we classified beneficiaries into the screening colonoscopy arm if they received a colonoscopy in the next seven days and into the no screening arm otherwise. To reduce computational time, we used a 5% random subsample of those in the no screening arm.
Next, using an approach previously described (25–29), we emulated a second trial with baseline a week after that of the first trial, and so on for every week while an individual was 70 years old. At the baseline week of each of these 52 sequential trials, eligibility criteria were reassessed. Beneficiaries who stopped meeting the eligibility criteria (e.g., because of a recent colonoscopy) were excluded from that trial; all others were reclassified into the two arms according to whether they had a colonoscopy during that week. We repeated the entire process for ages 71 to 79, which resulted in a total of 520 emulated trials. Each beneficiary may contribute as an eligible individual in as many trials as she is eligible for between the week she turns age 70 until the week she turns 80. Emulation of sequential trials is a valid and efficient procedure when beneficiaries can meet eligibility criteria at multiple times (25,30).
We also conducted analyses with an FOBT arm to assess the performance of our observational estimates against the published estimates from the FOBT randomized trials as a validity check for our method. We did not evaluate sigmoidoscopy because it was infrequently used in the Medicare population (eFigure 1 in the Supplementary material).
Outcomes
The primary outcome was CRC incidence. We also identified all adverse events occurring within 30 days after baseline that were severe enough to require an emergency department visit or hospitalization. We classified adverse events into “serious gastrointestinal events” (perforation, gastrointestinal bleeding requiring transfusion), “other gastrointestinal events” (gastrointestinal bleeding not requiring transfusion, paralytic ileus, nausea, vomiting and dehydration, abdominal pain), and “cardiovascular events” (myocardial infarction or angina, arrhythmias, congestive heart failure, cardiac or respiratory arrest, syncope, hypotension or shock) (21). We evaluated tumor stage among diagnosed CRC cases.
Statistical analysis
We pooled the individuals across all emulated trials and analyzed the data by age group (70–74, 75–79 years). We estimated curves for CRC cumulative incidence, both unadjusted and standardized to the baseline characteristics shown on Table 1: sex, race, age (linear and quadratic terms), original reason for entitlement, comprehensive preventive evaluation in the previous 2 years, use of 3 preventive services in the previous 2 years, census bureau division, combined comorbidity score, presence of each Chronic Condition Warehouse condition, calendar month. As in randomized trials, these curves estimate risks under hypothetical scenarios in which individuals do not die from causes other than CRC (16).
Table 1.
Baseline characteristics of eligible individuals by colonoscopy screening, Medicare 2004–2012
| Age 70–74 years | Age 75–79 years | |||
|---|---|---|---|---|
|
| ||||
| Colonoscopy n = 46,872 | No screening n = 1,762,816 | Colonoscopy n = 31,193 | No screening n = 1,628,020 | |
| Female, % | 50.5 | 49.6 | 50.4 | 50.1 |
| Race, % | ||||
| White | 93.0 | 92.1 | 93.4 | 92.5 |
| Black | 4.6 | 4.6 | 4.1 | 4.1 |
| Other | 2.5 | 3.3 | 2.5 | 3.4 |
| Age as original reason for entitlement*, % | 94.5 | 91.2 | 96.5 | 94.6 |
| Three preventive services in the previous 2 years | 14.1 | 9.2 | 12.3 | 7.9 |
| Census Bureau Division, % | ||||
| New England | 5.4 | 4.4 | 5.4 | 4.7 |
| Middle Atlantic | 8.4 | 9.6 | 9.0 | 10.1 |
| East North Central | 19.8 | 20.8 | 20.4 | 20.5 |
| West North Central | 12.6 | 10.4 | 12.9 | 10.5 |
| South Atlantic | 20.0 | 18.8 | 19.5 | 18.4 |
| East South Central | 7.1 | 8.0 | 6.7 | 7.6 |
| West South Central | 10.2 | 11.9 | 10.0 | 11.7 |
| Mountain | 7.6 | 6.8 | 7.2 | 6.6 |
| Pacific | 9.0 | 9.2 | 8.5 | 9.0 |
| Non-Census Bureau Division | 0.1 | 0.1 | 0.1 | 0.1 |
| Combined Comorbidity Score | ||||
| <0 | 20.5 | 20.2 | 21.5 | 20.5 |
| 0 | 67.6 | 65.8 | 63.3 | 62.3 |
| 1 | 8.6 | 9.4 | 10.7 | 11.0 |
| 2 | 2.0 | 2.6 | 2.7 | 3.4 |
| 3+ | 1.3 | 2.1 | 1.8 | 2.8 |
| CCW condition, % | ||||
| Alzheimer’s and related disorders | 2.5 | 3.9 | 4.2 | 7.2 |
| Acute myocardial infarction | 2.5 | 3.4 | 3.4 | 4.6 |
| Asthma | 8.9 | 9.3 | 9.7 | 9.8 |
| Atrial fibrillation | 7.6 | 8.9 | 11.2 | 12.9 |
| Cataract | 68.3 | 64.7 | 82.2 | 78.5 |
| Chronic heart failure | 11.1 | 15.7 | 16.5 | 21.4 |
| Chronic kidney disease | 7.2 | 9.2 | 9.6 | 11.6 |
| Endometrial cancer | 0.5 | 0.5 | 0.6 | 0.6 |
| Breast cancer | 3.3 | 2.9 | 3.8 | 3.5 |
| Lung cancer | 0.7 | 0.8 | 0.8 | 0.9 |
| Prostate cancer | 7.2 | 6.5 | 9.8 | 8.9 |
| COPD | 14.4 | 19.4 | 17.0 | 22.4 |
| Depression | 15.8 | 17.0 | 16.1 | 18.0 |
| Diabetes | 26.3 | 31.3 | 27.5 | 31.9 |
| Glaucoma | 19.8 | 18.8 | 24.8 | 23.4 |
| Hip/pelvic fracture | 0.7 | 0.8 | 1.1 | 1.6 |
| Hyperlipidemia | 79.3 | 77.8 | 81.4 | 79.6 |
| Benign prostatic hyperplasia | 21.3 | 20.2 | 27.8 | 26.0 |
| Hypertension | 74.9 | 78.0 | 80.5 | 82.7 |
| Hypothyroidism | 15.1 | 14.6 | 17.4 | 16.9 |
| Ischemic heart disease | 36.6 | 39.9 | 45.3 | 48.0 |
| Osteoporosis | 14.3 | 13.6 | 19.4 | 18.7 |
| Rheumatoid arthritis/osteoarthritis | 44.9 | 42.7 | 53.8 | 50.5 |
| Stroke | 6.6 | 8.5 | 9.6 | 12.2 |
Other possible reasons for entitlement are end-stage renal disease and disability.
CCW: chronic condition warehouse. COPD: chronic obstructive pulmonary disease.
To estimate the standardized curves, we fit a pooled logistic regression model for monthly CRC risk (31,32) that included an indicator for the screening arm, a flexible function of months of follow-up (linear, quadratic and exponentially decreasing term), product terms for arm and month, and the trial-specific baseline covariates (33) (detailed explanation in the Technical Appendix).
We also estimated the 30-day risk of adverse events standardized by age, sex and comorbidity score. We used a non-parametric bootstrap based on 500 individual-level resamplings to compute 95% confidence intervals. All analyses were conducted with SAS 9.4 (SAS Institute, Cary, NC). The Partners Human Research Committee and the Institutional Review Board at Harvard T.H. Chan School of Public Health approved our research.
Role of the Funding Source
This research was supported by the National Institutes of Health. The funders had no role in the study design, data collection and analysis, or decision to publish or preparation of the manuscript.
Results
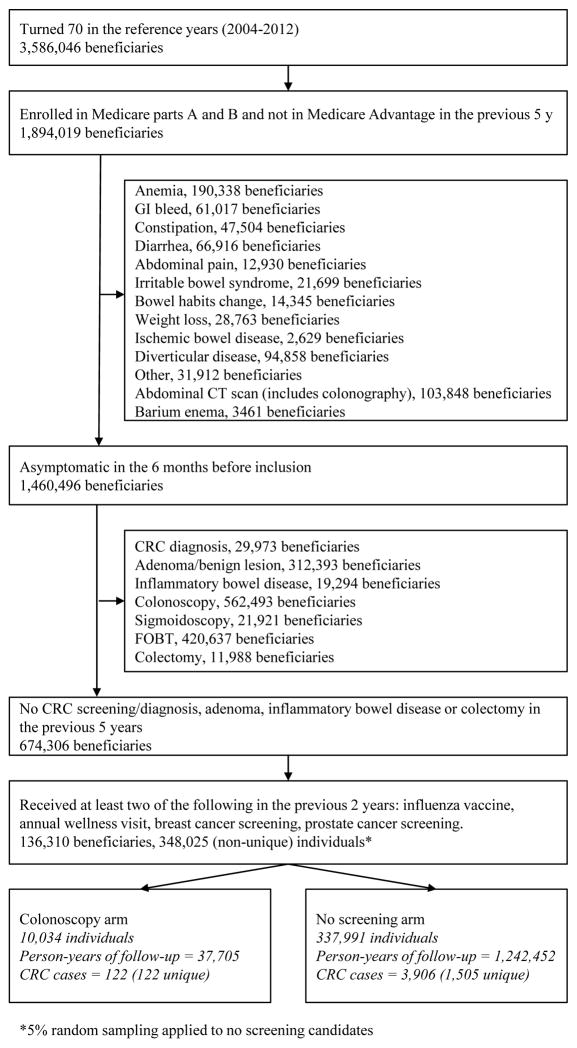
Of 3,586,046 Medicare beneficiaries reaching age 70 between the years 2004–2012, 674,306 had no previous CRC, were asymptomatic and without 5-year history of adenoma, inflammatory bowel disease, colectomy or screening. Of these, 136,310 were users of Medicare annual preventive services and thus eligible for our analyses. On average, each of these beneficiaries was eligible for 49.7 of the 52 emulated trials starting each week during the following year. After randomly selecting 5% of those in the no screening arm and pooling over all sequential trials, there were 348,025 (non-unique) individuals: 10,034 assigned to the screening colonoscopy arm and 337,991 to the no screening arm (Figure 1). The selection and assignment of beneficiaries aged 71 to 79 years is shown in the Supplementary material (eFigure 2). After pooling over all age groups, a total of 78,065 individuals were assigned to the screening colonoscopy arm, and 3,390,836 to the no screening arm. Median follow-up was 40 months (interquartile range 18–67 months).
Figure 1.
Flowchart of individuals aged 70 into the colonoscopy screening arms, Medicare 2004–2012.
Individual baseline characteristics were similar across the two arms, but the colonoscopy arm had a lower proportion of some chronic diseases (Alzheimer’s and related disorders, chronic heart failure, chronic obstructive pulmonary disease, diabetes, ischemic heart disease and stroke), a higher proportion of preventive services use in the 2 years before inclusion, and a higher proportion of some diagnoses (cataracts, benign prostatic hyperplasia and hyperlipidemia) that might reflect a higher utilization of health care services (Table 1).
Effectiveness of screening
During follow-up, there were 1,282 individuals diagnosed with CRC in the colonoscopy arm (685 aged 70–74, 597 aged 75–79) and 45,530 in the no screening arm (21,954 aged 70–74, 23,576 aged 75–79).
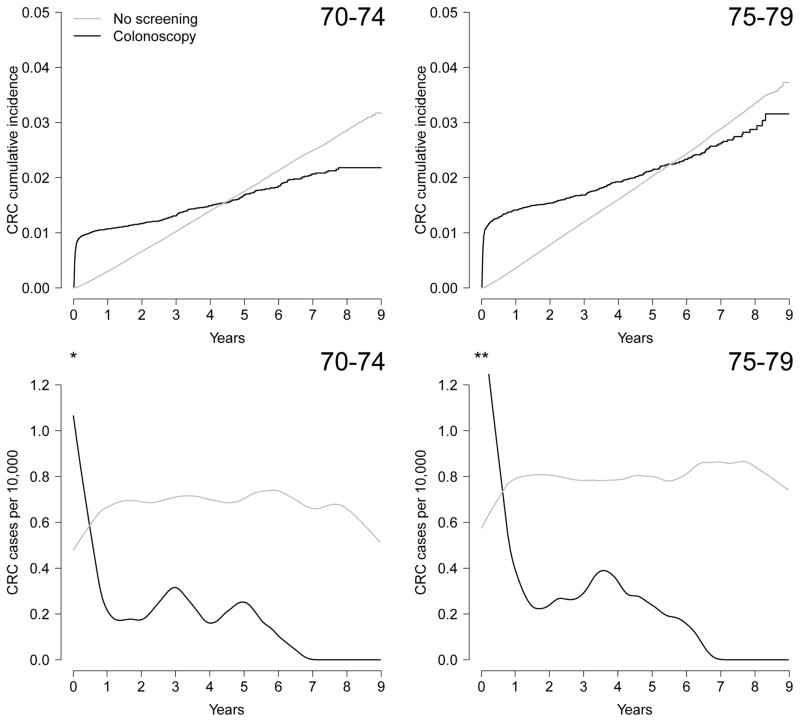
Because of detection of prevalent cancers at screening, the risk of CRC at baseline was higher in the colonoscopy arm (0.89% in the 70–74 age group and 1.14% in the 75–79 age group) than in the no screening arm (0.03% in both age groups), as expected. The curves crossed after about 4.5 years in the 70–74 age group and 5.5 years in the 75–79 age group, when the CRC risk became higher in the no screening arm (Figure 2). Adjustment for baseline covariates did not materially change the curves (eFigure 3 in the Supplementary material).
Figure 2.
Cumulative incidence and incidence rates of colorectal cancer by screening arm and age group, Medicare 2004–2012 (* Highest incidence is 32.8 CRC cases per 10,000 at week 2 after inclusion in the 70–74 age group. ** Highest incidence is 37.8 CRC cases per 10,000 at week 2 after inclusion in the 75–79 age group.)
In the 70–74 age group, the standardized 8-year risk of CRC (95% CI) was 2.19% (2.00, 2.37) in the colonoscopy screening arm and 2.62% (2.56, 2.67) in the no screening arm; risk difference −0.42% (−0.24, −0.63). In the 75–79 age group, the standardized 8-year risk of CRC (95% CI) was 2.84% (2.54, 3.13) in the colonoscopy screening arm and 2.97% (2.92, 3.03) in the no screening arm; risk difference −0.14% (−0.41, 0.16). Sensitivity analyses with different cutoff points for age (70–73 vs. 74–79 and 70–75 vs. 76–79) did not materially change results. A sensitivity analysis excluding enrollment from 2010–2012 (median follow-up, 58 months) yielded similar results: in the 70–74 age group the risk difference was −0.43% (−0.65, −0.21) and in the 75–79 age group the risk difference was −0.14% (−0.45, 0.18). Results did not vary by calendar time (eFigure 4).
In the subgroup of CRC cases that were linked to the SEER registry, 1,102 cases were diagnosed at screening colonoscopy and 24,969 without screening. The proportion of CRC cases in Stage 0 was 14.3% for screening colonoscopy vs. 8.1% for no screening, in Stage I 37.8% vs. 24.6%, in Stage II 19.1% vs. 26.7%, in Stage III 22.1% vs. 24.0 and in Stage IV 6.7% vs. 16.7%. Results were similar across age groups (eTable 3 in the Supplementary material).
We also evaluated colonoscopy-based surveillance after the initial screening. The use of colonoscopy peaks at years 3 and 5 among individuals in whom the screening colonoscopy removed a polyp, and is very low until year 5 among those in whom the screening colonoscopy that did not find a polyp. These patterns were present in both age groups (eFigure 5 in the Supplementary material).
In analyses that compared FOBT versus no screening, the CRC risk in the FOBT arm was always higher than in the no screening arm because of the detection of some (but not all) prevalent cancers at baseline (eTable 2 and eFigure 3 in the Supplementary material), as expected. Individuals in the FOBT arm frequently received a colonoscopy shortly after inclusion into the study, presumably due to a positive FOBT result.
Safety of Screening
Compared with the no screening arm, the excess 30-day risk of any adverse event requiring hospitalization or a visit to the emergency department in the colonoscopy arm was 5.6 adverse events per 1,000 individuals (95% CI 4.4–6.8) in the 70–74 age group and 10.3 adverse events per 1,000 individuals (95% CI 8.6–11.1) in the 75–79 group. The increased risk for each individual adverse event was low (less than 2 cases per 1,000 persons), except for arrhythmia, with an excess risk of 2.4 cases per 1,000 individuals (95% CI 1.6–3.2) in the 70–74 age group and of 5.5 cases per 1,000 individuals (95% CI 4.4–6.9) in the 75–79 age group (Table 2).
Table 2.
Standardized risk, per 1000 individuals, of adverse events requiring an admission or an emergency room visit within 30 days of colonoscopy screening, Medicare 2004–2012.
| Ages 70–74 | Ages 75–79 | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Colonoscopy | No screening | Colonoscopy | No screening | |||||
|
| ||||||||
| n | Risk (95% CI) | n | Risk (95% CI) | n | Risk (95% CI) | n | Risk (95% CI) | |
| Any adverse event | 691 | 15.4 (14.2–16.7) | 17,161 | 9.7 (9.6–9.9) | 691 | 23.2 (21.7–25.0) | 20,982 | 12.9 (12.7–13.0) |
| Serious GI events | 40 | 0.9 (0.6–1.2) | 181 | 0.1 (0.1–0.1) | 25 | 0.8 (0.5–1.2) | 250 | 0.2 (0.1–0.2) |
| Perforations | 20 | 0.4 (0.2–0.6) | 51 | 0 (0–0) | 11 | 0.4 (0.2–0.6) | 71 | 0 (0–0.1) |
| GI bleeding requiring transfusion | 20 | 0.4 (0.3–0.7) | 130 | 0.1 (0.1–0.1) | 14 | 0.5 (0.2–0.7) | 180 | 0.1 (0.1–0.1) |
| Other GI events | 257 | 5.6 (4.9–6.2) | 4,331 | 2.5 (2.4–2.5) | 206 | 6.9 (6.0–7.7) | 5,003 | 3.2 (3.1–3.3) |
| GI bleeding not requiring transfusion | 55 | 1.2 (0.9–1.6) | 344 | 0.2 (0.2–0.2) | 48 | 1.6 (1.2–2.2) | 448 | 0.3 (0.3–0.3) |
| Paralytic ileus | 41 | 0.9 (0.6–1.2) | 266 | 0.2 (0.1–0.2) | 41 | 1.3 (0.9–1.8) | 290 | 0.2 (0.2–0.2) |
| Nausea and vomiting, dehydration | 100 | 2.2 (1.8–2.6) | 2,377 | 1.3 (1.3–1.4) | 86 | 2.9 (2.3–3.5) | 2,972 | 1.8 (1.8–1.9) |
| Abdominal pain | 71 | 1.5 (1.2–1.9) | 1,691 | 1.0 (0.9–1.0) | 43 | 1.4 (1.0–1.8) | 1,679 | 1.0 (1.0–1.1) |
| Cardiovascular events | 473 | 10.7 (9.7–11.6) | 14,026 | 8.4 (8.2–8.5) | 538 | 18.1 (16.8–19.7) | 17,638 | 10.8 (10.7–11.0) |
| MI or angina | 66 | 1.5 (1.1–1.9) | 2,957 | 1.7 (1.6–1.7) | 82 | 2.7 (2.2–3.3) | 3,274 | 2.0 (1.9–2.1) |
| Arrhythmias | 297 | 6.8 (6.0–7.6) | 7,752 | 4.4 (4.3–4.5) | 351 | 12.0 (10.8–13.3) | 10,471 | 6.4 (6.3–6.6) |
| Congestive heart failure | 124 | 2.9 (2.4–3.4) | 4,709 | 2.7 (2.6–2.7) | 160 | 5.6 (4.8–6.4) | 5,951 | 3.7 (3.6–3.8) |
| Cardiac or respiratory arrest | 30 | 0.7 (0.4–0.9) | 572 | 0.3 (0.3–0.3) | 26 | 0.9 (0.5–1.2) | 654 | 0.4 (0.4–0.5) |
| Syncope, hypotension/shock | 117 | 2.6 (2.1–3.1) | 3,026 | 1.7 (1.7–1.8) | 118 | 3.9 (3.2–4.6) | 3,828 | 2.4 (2.3–2.4) |
GI: gastrointestinal, MI: myocardial infarction.
Discussion
We estimated that screening colonoscopy reduces the eight-year risk of CRC from approximately 2.6% to 2.2% in beneficiaries aged 70 to 74 years, and from 3.0% to 2.8% in those aged 75 to 79 years. The excess risk of serious adverse events after colonoscopy was small, especially among younger beneficiaries.
Our findings are consistent with the USPSTF recommendations for routine screening through age 75, followed by individualized decisions afterwards (17). Because the ongoing trials (3–5) do not include the older age groups (eTable 1), our study provides helpful information for benefit-risk analyses. Our estimates of the impact of screening colonoscopies on CRC incidence and complication rates in older persons are particularly important in view of current policies to increase screening uptake: the Healthy People 2020 goal is a 70 percent CRC screening rate (34).
Based on Medicare surveillance patterns (eFigure 5 in the Supplementary material), our screening colonoscopy arm corresponds approximately to the strategy “receive a screening colonoscopy; if a polyp is detected, repeat at 3 or 5 years; if no polyp is detected, repeat at 5 years or later”. Our estimates are not directly comparable with previous observational analyses of colonoscopy and CRC incidence which were based on comparisons that are less relevant for decision-making (e.g., “receiving a negative colonoscopy” or “polypectomy” versus “no lower endoscopy” (35)), were mostly restricted to younger age groups, and did not estimate absolute risks (36–40).
The follow-up of our study—25% of beneficiaries were followed more than 5.5 years—may be insufficient to detect the full benefits of screening colonoscopy, although our estimates suggest a greater CRC absolute risk reduction at 8 years than that found by the screening sigmoidoscopy trials (11,12,14,15), especially in the younger age group. Though the absence of cause of death information in Medicare data precludes the evaluation of CRC-specific mortality, we would expect that both CRC-mortality and morbidity be improved in the screening arm. In addition to the lower CRC incidence, over half of the CRC cases detected through screening colonoscopy were stage 0 or I, compared with a third of interval cancers. Because Stages 0 and I have an excellent prognosis and do not require adjuvant chemotherapy, earlier detection by screening contributes to better cancer-specific survival and better quality of life. A concern of population-based cancer screening programs is overdiagnosis (41,42) and treatment of indolent cancer that would never become symptomatic. Screening colonoscopy, however, identifies and removes precancerous lesions, which do not require further treatment, and is a low risk procedure (as opposed to, for example, removing a pulmonary node detected in screening low-dose CT scan). Therefore, screening colonoscopy might face fewer challenges than other screening programs.
Like in any observational study, our estimates might be confounded by unmeasured risk factors for CRC. However, in this case, the are several reasons to doubt that substantial confounding exists. First, our estimates are consistent with those of observational analyses of three sigmoidoscopy randomized trials (11,12,14) that ignored randomization by comparing the CRC incidence between the control group with the non-compliers in the intervention group. The three trials consistently found no differences in CRC incidence, which suggests little, if any, confounding, that is, CRC risk factors seem to be little associated with the reasons why individuals decide to undergo CRC screening. Second, previous observational studies have found very little impact on the effect estimates after adjustment for potential confounders (39,43,44). Third, our FOBT estimates in the younger age group were compatible with the benchmark provided by FOBT trials (7,45), which further supports the validity of our approach to emulate a CRC screening randomized trial. Sensitivity analyses (46) confirm that the conclusions of our study would not change under realistic scenarios of unmeasured confounding (eFigure 6). In contrast, as in the observational analyses of the sigmoidoscopy trials, we suspect substantial confounding exists for the effect of CRC screening on all-cause mortality (16) (eFigure 7 in the Supplementary material), for which unmeasured lifestyle prognostic factors such as cigarette smoking are more relevant.
Further, we included only users of Medicare preventive services to reduce confounding and to increase the specificity of our classification of colonoscopies as screening tests. This selection might reduce external validity, as it happens in any clinical trial that applies a set of eligibility criteria, but increases internal validity by reducing the differences between arms with respect to measured variables (data not shown) and therefore probably with respect to unmeasured variables too. A sensitivity analysis without this selection yields the same effect estimate, but an implausibly high cancer prevalence (eFigure 8), possibly due to the inclusion of colonoscopies conducted for diagnostic purposes, which supports our selection as a strategy to reduce misclassification of screening colonoscopies. Thus the slightly higher prevalence of CRC at the baseline colonoscopy in our study compared with that reported in two ongoing randomized trials (3,47) and several observational studies (48–50), likely results from a higher prevalence of asymptomatic CRC in our older population rather than from misclassification of diagnostic colonoscopies. We also found a higher baseline prevalence of CRC in our FOBT arm (eFigure 3 in the Supplementary material) than in published FOBT studies (7,45), and FOBT is rarely used for reasons other than screening.
In summary, we provide precise estimates of the effectiveness and safety of screening colonoscopy in individuals aged 70 and older, an underrepresented population in randomized trials. Our findings suggest a modest benefit of screening colonoscopy for the prevention of CRC in persons aged 70–74 years, and a smaller (if any) benefit in older individuals. The risk of adverse events was low in both age groups. Our findings can assist patients, physicians, and policy makers make informed decisions about CRC screening.
Supplementary Material
Acknowledgments
Funding source: NIH grants P01-CA134294, R01-CA164023, R01-HS023128.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015 Jan 5;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman Da, Rex DK, Winawer SJ, Giardiello FM, Johnson Da, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012 Sep;143(3):844–57. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas Á, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012 Feb 23;366(8):697–706. doi: 10.1056/NEJMoa1108895. [DOI] [PubMed] [Google Scholar]
- 4.Kaminski MF, Bretthauer M, Zauber AG, Kuipers EJ, Adami H-O, van Ballegooijen M, et al. The NordICC Study: rationale and design of a randomized trial on colonoscopy screening for colorectal cancer. Endoscopy. 2012 Jul;44(7):695–702. doi: 10.1055/s-0032-1306895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colonoscopy Versus Fecal Immunochemical Test in Reducing Mortality From Colorectal Cancer (CONFIRM) [Internet] [cited 2015 Jun 3]. Available from: https://clinicaltrials.gov/ct2/show/NCT01239082.
- 6.Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013 Sep 19;369(12):1106–14. doi: 10.1056/NEJMoa1300720. [DOI] [PubMed] [Google Scholar]
- 7.Scholefield JH, Moss SM, Mangham CM, Whynes DK, Hardcastle JD. Nottingham trial of faecal occult blood testing for colorectal cancer: a 20-year follow-up. Gut. 2011 Nov 3;61(7):1036–40. doi: 10.1136/gutjnl-2011-300774. [DOI] [PubMed] [Google Scholar]
- 8.Kronborg O, Jørgensen OD, Fenger C, Rasmussen M. Randomized study of biennial screening with a faecal occult blood test: results after nine screening rounds. Scand J Gastroenterol. 2004 Sep;39(9):846–51. doi: 10.1080/00365520410003182. [DOI] [PubMed] [Google Scholar]
- 9.Faivre J, Dancourt V, Lejeune C, Tazi MA, Lamour J, Gerard D, et al. Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology. 2004 Jun;126(7):1674–80. doi: 10.1053/j.gastro.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Lindholm E, Brevinge H, Haglind E. Survival benefit in a randomized clinical trial of faecal occult blood screening for colorectal cancer. Br J Surg. 2008 Aug;95(8):1029–36. doi: 10.1002/bjs.6136. [DOI] [PubMed] [Google Scholar]
- 11.Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JMA, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010 May 8;375(9726):1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 12.Segnan N, Armaroli P, Bonelli L, Risio M, Sciallero S, Zappa M, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial--SCORE. J Natl Cancer Inst. 2011 Sep 7;103(17):1310–22. doi: 10.1093/jnci/djr284. [DOI] [PubMed] [Google Scholar]
- 13.Hoff G, Grotmol T. Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: randomised controlled trial. BMJ. 2009:4–10. doi: 10.1136/bmj.b1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holme Ø, Løberg M, Kalager M, Bretthauer M, Hernán MA, Aas E, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA. 2014 Aug 13;312(6):606–15. doi: 10.1001/jama.2014.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012 Jun 21;366(25):2345–57. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holme Ø, Bretthauer M, Fretheim A, Odgaard-Jensen J, Hoff G. Flexible sigmoidoscopy versus faecal occult blood testing for colorectal cancer screening in asymptomatic individuals. Cochrane database Syst Rev. 2013 Jan;9:CD009259. doi: 10.1002/14651858.CD009259.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Preventive Services Task Force. Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016 Jun 21;315(23):2564–75. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- 18.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009 Mar;104(3):739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 19.NCCN Clinical Practice Guidelines in Oncology. Colorectal Cancer Screening Version 1.2015 [Internet] [cited 2015 Nov 30]. Available from: http://www.nccn.org/professionals/physician_gls/PDF/colorectal_screening.pdf.
- 20.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011 Jul;64(7):749–59. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warren JL, Klabunde CN, Mariotto AB, Meekins A, Topor M, Brown ML, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009 Jun 16;150(12):849–57. W152. doi: 10.7326/0003-4819-150-12-200906160-00008. [DOI] [PubMed] [Google Scholar]
- 22.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002 Aug;40(8 Suppl):IV, 3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 23.Goodwin JS, Singh A, Reddy N, Riall TS, Kuo Y-F. Overuse of screening colonoscopy in the Medicare population. Arch Intern Med. 2011 Aug 8;171(15):1335–43. doi: 10.1001/archinternmed.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ackermann RT, Williams B, Nguyen HQ, Berke EM, Maciejewski ML, LoGerfo JP. Healthcare cost differences with participation in a community-based group physical activity benefit for medicare managed care health plan members. J Am Geriatr Soc. 2008 Aug;56(8):1459–65. doi: 10.1111/j.1532-5415.2008.01804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernán MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am J Epidemiol. 2016 Apr 15;183(8):758–64. doi: 10.1093/aje/kwv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danaei G, Rodríguez LAG, Cantero OF, Logan R, Hernán MA. Observational data for comparative effectiveness research: an emulation of randomised trials of statins and primary prevention of coronary heart disease. Stat Methods Med Res. 2013 Feb;22(1):70–96. doi: 10.1177/0962280211403603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernan M, Robins J, Garcia-Rodriguez L. Discussion on “Statistical Issues in the Women’s Health Initiative”. Biometrics. 2005;61(4):922–30. doi: 10.1111/j.0006-341X.2005.454_1.x. [DOI] [PubMed] [Google Scholar]
- 28.Hernán MA, Alonso A, Logan R, Grodstein F, Michels KB, Willett WC, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008 Nov;19(6):766–79. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stampfer MJ. ITT for observational data: worst of both worlds? Epidemiology. 2008 Nov;19(6):783–4. doi: 10.1097/EDE.0b013e318188442e. discussion 789–93. [DOI] [PubMed] [Google Scholar]
- 30.Hernán M, Sauer B, Hernández-Díaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol. 2016 doi: 10.1016/j.jclinepi.2016.04.014. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson WA. On the treatment of grouped observations in life studies. Biometrics. 1977 Sep;33(3):463–70. [PubMed] [Google Scholar]
- 32.D’Agostino RB, Lee ML, Belanger AJ, Cupples LA, Anderson K, Kannel WB. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat Med. 1990 Dec;9(12):1501–15. doi: 10.1002/sim.4780091214. [DOI] [PubMed] [Google Scholar]
- 33.Hernán MA. The hazards of hazard ratios. Epidemiology. 2010 Jan;21(1):13–5. doi: 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.U.S. Department of Health and Human Services. Healthy People 2020 Objectives, Objective C-16 [Internet] [cited 2015 Dec 23]. Available from: http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=5.
- 35.Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, et al. Long-Term Colorectal-Cancer Incidence and Mortality after Lower Endoscopy. N Engl J Med. 2013 Sep 19;369(12):1095–105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009 Jan 6;150(1):1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 37.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011 Jan 4;154(1):22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 38.Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ. 2014 Jan;348:g2467. doi: 10.1136/bmj.g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kahi CJ, Myers LJ, Slaven JE, Haggstrom D, Pohl H, Robertson DJ, et al. Lower endoscopy reduces colorectal cancer incidence in older individuals. Gastroenterology. 2014;146(3):718–25. doi: 10.1053/j.gastro.2013.11.050. [DOI] [PubMed] [Google Scholar]
- 40.Stock D, Paszat LF, Rabeneck L. Colorectal cancer mortality reduction is associated with having at least 1 colonoscopy within the previous 10 years among a population-wide cohort of screening age. Gastrointest Endosc. 2016;84(1):133–41. doi: 10.1016/j.gie.2015.12.035. [DOI] [PubMed] [Google Scholar]
- 41.Shieh Y, Eklund M, Sawaya GF, Black WC, Kramer BS, Esserman LJ. Population-based screening for cancer: hope and hype. Nat Rev Clin Oncol. 2016 doi: 10.1038/nrclinonc.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esserman LJ, Thompson IM, Reid B, Nelson P, Ransohoff DF, Welch HG, et al. Addressing overdiagnosis and overtreatment in cancer: A prescription for change. Lancet Oncol. 2014;15(6):e234–42. doi: 10.1016/S1470-2045(13)70598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doubeni CA, Weinmann S, Adams K, Kamineni A, Buist DSM, Ash AS, et al. Screening colonoscopy and risk for incident late-stage colorectal cancer diagnosis in average-risk adults: a nested case-control study. Ann Intern Med. 2013 Mar 5;158(5 Pt 1):312–20. doi: 10.7326/0003-4819-158-5-201303050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brenner H, Chang-Claude J, Jansen L, Knebel P, Stock C, Hoffmeister M. Reduced risk of colorectal cancer up to 10 years after screening, surveillance, or diagnostic colonoscopy. Gastroenterology. 2014;146(3):709–17. doi: 10.1053/j.gastro.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993 May 13;328(19):1365–71. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 46.Ding P, VanderWeele TJ. Sensitivity Analysis Without Assumptions. Epidemiology. 2006;27(3):368–77. doi: 10.1097/EDE.0000000000000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bretthauer M, Kaminski MF, Løberg M, Zauber AG, Regula J, Kuipers EJ, et al. Population-Based Colonoscopy Screening for Colorectal Cancer: A Randomized Clinical Trial. JAMA Intern Med. 2016 Jul 1;176(7):894–902. doi: 10.1001/jamainternmed.2016.0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bokemeyer B, Bock H, Hüppe D, Düffelmeyer M, Rambow A, Tacke W, et al. Screening colonoscopy for colorectal cancer prevention: results from a German online registry on 269000 cases. Eur J Gastroenterol Hepatol. 2009 Jun;21(6):650–5. doi: 10.1097/meg.0b013e32830b8acf. [DOI] [PubMed] [Google Scholar]
- 49.Stoop EM, de Haan MC, de Wijkerslooth TR, Bossuyt PM, van Ballegooijen M, Nio CY, et al. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: A randomised controlled trial. Lancet Oncol. 2012;13(1):55–64. doi: 10.1016/S1470-2045(11)70283-2. [DOI] [PubMed] [Google Scholar]
- 50.Pox CP, Altenhofen L, Brenner H, Theilmeier A, Von Stillfried D, Schmiegel W. Efficacy of a nationwide screening colonoscopy program for colorectal cancer. Gastroenterology. 2012;142(7):1460–7.e2. doi: 10.1053/j.gastro.2012.03.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.