Abstract
In ruminants, pulsatile release of prostaglandin F2α (PGF2α) from the endometrium is transported to the ovary and induces luteolysis thereby allowing new estrous cycle. Interferon tau (IFNT), a type 1 IFN secreted by the trophoblast cells of the developing conceptus, acts on endometrial luminal epithelial (LE) cells and inhibits pulsatile release of PGF2α and establishes pregnancy. One of the unknown mechanisms is that endometrial pulsatile release of PGF2α is inhibited whereas basal release of PGF2α is increased in pregnant compared with nonpregnant sheep. We have recently found that pulsatile release of PGF2α from the endometrium is regulated by prostaglandin transporter (PGT)-mediated mechanisms. We hypothesize that modulation in the endometrial pulsatile vs. basal release of PGF2α likely requires PGT-mediated selective transport, and IFNT interacts with PGT protein and modulates pulsatile vs. basal release of PGF2α. The new findings of the present study are: 1) IFNT activates novel JAK-SRC kinase-EGFR-RAS-RAF-ERK1/2-early growth response (EGR)-1 signaling module in LE cells; 2) IFNT increases interactions between PGT and ERK1/2 or EGR-1 proteins and alters phosphorylation of PGT protein; 3) IFNT precludes action of protein kinase C and Ca2+ on PGT function; and 4) IFNT inhibits 80% PGT-mediated but not 20% simple diffusion-mediated release of PGF2α from the endometrial LE cells through this novel signaling module. The results of the present study provide important new insights on IFNT signaling and molecular control of PGT-mediated release of PGF2α and unravel the underlying mechanisms responsible for the increased basal release of PGF2α at the time of establishment of pregnancy in ruminants.
Interferon tau inhibits prostaglandin transporter (PGT)-mediated pulsatile release of PGF2α by the endometrial epithelial cells through novel JAK-SRC-EGFR-RAS-RAF-ERK1/2-EGR-1 signaling module in ruminants.
It is well accepted that pulsatile release of prostaglandin F2α (PGF2α) from the endometrial luminal epithelial cells (LE) and superficial glandular epithelial cells (sGLE) regresses corpus luteum (luteolysis) in ruminants (1, 2). In sheep, continuous exposure of endometrium to progesterone (P4) for 8–10 d down-regulates expression of nuclear P4 receptor (PGR) in LE and sGLE cells between d 11–d 13, thereby allowing a rapid increase in expression of nuclear estrogen receptor α (ESR1) after d 13 followed by an increase in expression of membrane oxytocin receptor (OXTR) after d 14 of the estrous cycle (1, 2, 3). Pulsatile release of oxytocin (OT) from the posterior pituitary after d 13–d 14 of the estrous cycle acts on endometrial OXTR and induces release of luteolytic pulses of PGF2α from the endometrial LE and sGE cells between d 14 and d 16 of the estrous cycle (2). PGF2α released from LE and sGLE is effluxed into the utero-ovarian vein and transported to the ovarian artery via the vascular utero-ovarian plexus to induce luteal regression (1). We have recently found that transport of PGF2α from the endometrium to the ovary is facilitated by prostaglandin transporter (PGT) protein-mediated mechanisms in ruminants (4, 5, 6).
Endometrial production of PGF2α peaks on d 14–d 15 after estrus in both pregnant and nonpregnant sheep (7, 8). Nonpregnant sheep display pulsatile release of PGF2α superimposed on a constant baseline whereas pregnant sheep show increased continuous basal release of PGF2α (7). During the period of maternal recognition of pregnancy, one of the fascinating aspects is that pulsatile release of PGF2α is inhibited; by contrast, the basal concentrations of PGF2α are increased in pregnant compared with nonpregnant sheep (7, 8). Results of these early studies suggest that inhibition of pulsatile release of PGF2α from the endometrial LE and sGLE at the time of recognition of pregnancy in sheep might not exclusively be regulated by either biosynthesis of PGF2α or signaling of OXTR. This notion is supported by the fact that: 1) COX-2 protein is abundantly expressed in LE and sGLE of the endometrium on d 12–d 16 of the estrous cycle and pregnancy (9, 10); and 2) despite the absence of OXTR in LE and sGLE, the endometrium has innate ability to produce large amounts of basal PGF2α in an OXTR-independent manner at the time of recognition of pregnancy (7, 8). These results may suggest that modification in the pulsatile release PGF2α at the time of recognition of pregnancy vs. luteolysis would be the key mechanism. It likely requires selective PGT-mediated transport and cannot be supported by a simple diffusion mechanism. In our previous studies, we have found that 80% of PGF2α release is facilitated by a PGT-mediated mechanism and 20% of PGF2α release is facilitated by a PGT-independent simple diffusion mechanism in endometrial LE cells in vitro, and inhibition of PGT suppressed OT-induced and PGT-mediated release of PGF2α from the ovine endometrium in vivo at the time of luteolysis (5, 6).
In ruminants, during establishment of pregnancy either IFNT or interferon tau (IFNT), a type 1 interferon secreted by the mononuclear cells of trophectoderm of the conceptus, acts on LE and sGE cells in a paracrine manner and suppresses transcription of ESR1 and OXTR genes (2, 3) and thereby inhibits OT-induced pulsatile release of luteolytic PGF2α by the endometrium. This antiluteolytic mechanism of IFNT is involved through IFN-regulatory factor (IRF)-2, a potent repressor of gene transcription (11). IFNΤ signaling is mediated through cell surface receptor that is composed of two subunits IFNAR1 and IFNAR2. The well-characterized downstream signal transduction pathways of type I IFN is activation of tyrosine kinase 2 and janus kinase 1 (JAK1) and signal transducer and activator of transcription (STAT) collectively known as the JAK/STAT pathways (12, 13). Activation of JAK/STAT, in turn, regulates phosphorylation and activation of STATs that forms homodimers or heterodimers that activate IFN-stimulated genes (ISGs). Phosphorylated STAT1 and STAT2 (heterodimers) together with interferon regulatory factor-9 (IRF-9) form IFN-stimulated gene factor 3 transcriptional complex that translocates to the nucleus and binds with IFN-stimulated response elements to initiate transcription of specific ISGs. Interestingly, IFNAR1 and IFNAR2 genes are expressed (14) but STAT1, STAT2 and IRF-9 genes are not expressed in endometrial LE and sGLE cells at the time of recognition of pregnancy in sheep (3, 11). On the other hand, IFNΤ-stimulated genes are expressed in LE and sGLE cells (15). This suggests that IFNT can apparently activate STAT-independent cell-signaling pathways in endometrial LE and sGLE cells at the time of establishment of pregnancy (3).
It is well established that the antiluteolytic mechanism of IFNT is mediated through IRF2-ESR1-OXTR pathways in endometrial LE and sGLE cells at the time of recognition of pregnancy in ruminants (2, 3). However, accumulating current cellular and molecular information on PGT-mediated transport of PGF2α (4, 5, 6) and requirement for selective modulation in the pulsatile release of PGF2α at the time of recognition of pregnancy (7, 8) lead us to identify novel mechanisms. We hypothesize that modulation in the pulsatile vs. basal release of PGF2α likely requires PGT-mediated selective transport and IFNT interacts with PGT protein through novel cell- signaling pathways and thereby inhibits PGT-mediated release but not basal release of PGF2α from the endometrial LE cells. Therefore, the objective of the present study was to determine molecular mechanisms through which IFNT regulates PGT-mediated release of PGF2α from the endometrial LE cells. We used sheep as a ruminant model. Results of the present study for the first time provide direct evidence that IFNT inhibits PGT-mediated release of PGF2α from the ovine endometrial LE cells through novel JAK-SRC kinase (SRC)-EGFR-RAS-RAF-ERK-early growth factor (EGR)-1 pathways.
Results
IFNT inhibited release, but not uptake, of PGF2α by the ovine endometrial LE cells
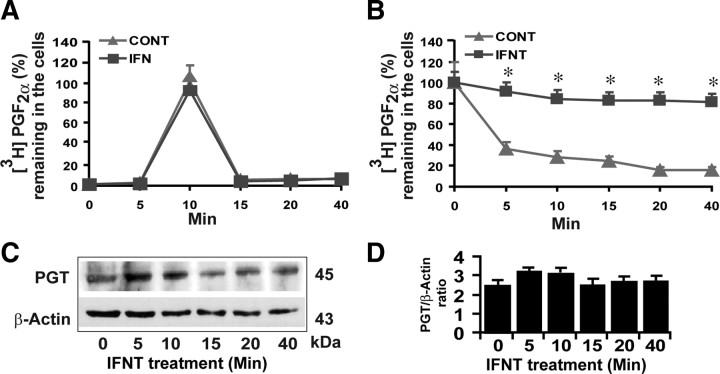
Results indicated that uptake of [3H]PGF2α peaked (P < 0.05) at 10 min and then decreased to basal levels by 15–20 min (Fig. 1A) in LE cells. Release of [3H]PGF2α up to 80% occurred rapidly (P < 0.05) during the first 5 to 10 min leaving less than 20% of [3H]PGF2α inside the cells and reached basal levels within 40 min (Fig. 1B) in LE cells. Interestingly, IFNT inhibited (P < 0.05) 80% of release but not uptake of [3H]PGF2α by the LE cells. These results, together with our previous study (5), strongly indicate that 80% release of PGF2α is mediated by PGT and the other 20% of PGF2α release is facilitated by simple diffusion in LE cells. We then validated this functional data by examining effect of IFNT on the expression of PGT protein. Western blot analyses (Fig. 1, C and D) indicated that IFNT neither decreased nor increased expression of PGT protein in LE cells within 0–40 min. These data would suggest that the function of PGT protein can be modulated by IFNT-induced rapid intracellular signaling pathways. Therefore, we used PGF2α uptake-release LE cell system as a valuable tool to determine novel molecular mechanism through which IFNT inhibits PGT-mediated release of PGF2α by the ovine endometrium.
Fig. 1.
Effects of IFNT on PGT-mediated transport of PGF2α in the ovine endometrial LE cells determined by influx/uptake and efflux/release experiments as described in Materials and Methods. A, Uptake of PGF2α by the LE cells. B, Release of PGF2α by the LE cells. C, Western blot analysis of PGT protein and β-actin protein as the internal control. D, Densitometry was determined by Alpha Imager, and relative expressions were expressed based on the integrated density values. CONT, Control. *, CONT vs. IFNT, P < 0.05. All numerical values are expressed as mean ± sem of three (n = 3) independent experiments.
IFNT inhibited release of PGF2α through EGFR-ERK1/2-EGR-1 signaling pathways in LE cells
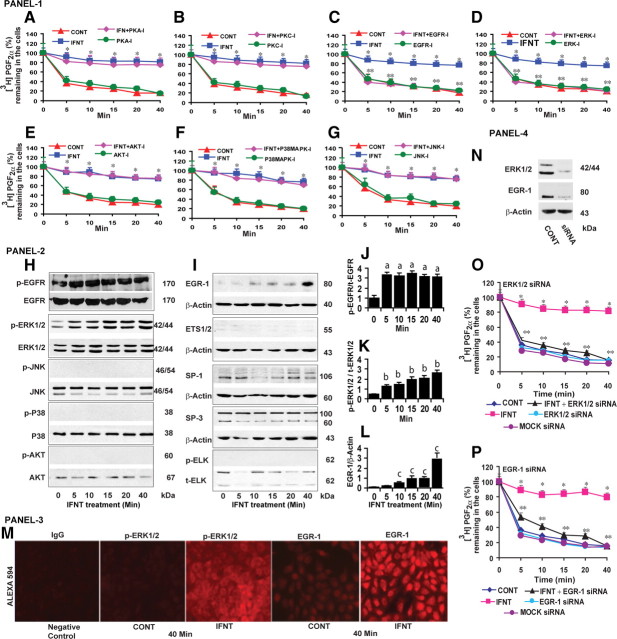
In our previous study (5), we reported the presence of multiple sites for various serine, threonine, and tyrosine protein kinases in the extracellular, intracellular, and transmembrane domains of ovine PGT protein. Based on this information, we evaluated roles for protein kinase A (PKA), protein kinase C (PKC), and epidermal growth factor receptor (EGFR) pathways on inhibitory effects of IFNT on PGT-mediated release of PGF2α in LE cells. Results indicated that inhibition of PKA and PKC (Fig. 2, A and B) pathways did not reverse by contrast inhibition of EGFR pathways (Fig. 2C) reversed (P < 0.05) the effects of IFNT on 80% PGT-mediated release of PGF2α. Downstream of EGFR, inhibition of ERK1/2 reversed (P < 0.05) the inhibitory effects of IFNT on 80% PGT-mediated release of PGF2α, and inhibition of PI3K/AKT, P38MAPK, and JNK pathways did not have any effect (Fig. 2, D–G). Inhibition of PKA, PKC, EGFR, ERK1/2, PI3K/AKT, P38MAPK and JNK/SAPK pathways did not show any effect on PGT-mediated release of PGF2α in the absence of IFNT. We then confirmed this functional data by examining the effect of IFNT on activation or inactivation of EGFR downstream pathway proteins by Western blot. Results (Fig. 2, H–L) indicated that IFNT phosphorylated (P < 0.05) EGFR and ERK1/2 proteins between 5 and 40 min and did not phosphorylate AKT, P38MAPK, and JNK proteins. Furthermore, IFNT increased (P < 0.05) expression of EGR-1 protein between 10 and 40 min but not ETS1/2, SP1, SP3, and p-ELK proteins. This temporal phosphorylation/activation of EGFR, ERK1/2, and EGR-1 proteins was positively correlated (r = +0.93) with IFNT-inhibited and PGT-mediated release of PGF2α in LE cells. In addition, our immunofluorescence analysis (Fig. 2M) confirmed that IFNT phosphorylated (P < 0.05) ERK1/2 protein and increased (P < 0.05) expression of EGR-1 protein in the majority of the LE cells within 40 min. To confirm the observed pharmacological findings, we then knocked down ERK1/2 or EGR-1 genes. Results (Fig. 2, N–P) indicated that loss of function of ERK1/2 and EGR-1 reversed (P < 0.05) the inhibitory effects of IFNT on 80% PGT-mediated release of PGF2α by the LE cells. Collectively, these results indicate that IFNT inhibits PGT-mediated release of PGF2α through a novel EGFR-ERK1/2-EGR-1 signaling module in the ovine endometrial LE cells.
Fig. 2.
Panel 1 (A–G), Effects of PKA, PKC, EGFR, ERK1/2, AKT, P38MAPK, JNK pathways on inhibitory effects of IFNT on PGT-mediated release of PGF2α from the LE cells. CONT, Control. Inhibitors for PKA (H-89, 50 nm), PKC (GF109203, 10 μm), EGFR (AG1478, 15 μm), ERK1/2 (U0126, 10 μm), PI3K/AKT (LY294002, 50 μm), P38MAPK (SB203580, 10 μm), and JNK/SAPK (SP600125, 10 μm) were indicated by PKA-I, PKC-I, EGFR-I, ERK-I, PI3K-I, AKT-I, P38MAPK-I, and JNK-I, respectively. *, CONT vs. IFNT, P < 0.05. **, IFNT vs. IFNT+ EGFR-I or IFNT+ERK-I, P < 0.05. Panel 2 (H–I), Effects of IFNT on activation/phosphorylation of EGFR, ERK1/2, AKT, P38MAPK, JNK, EGR-1, ETS1/2, SP1, SP3, and ELK proteins, analyzed by Western blot. Total form of respective protein or β-actin protein was measured as internal control. J–L, Densitometry was determined by Alpha Imager, and relative expressions were expressed based on the integrated density values. Effects of IFNT on (a) p-EGFR protein at 0 vs. 5–40 min, (b) on p-ERK1/2 protein at 0 vs. 5–40 min, and (c) on EGR-1 protein at 0 vs. 10–40 min, P < 0.05. Panel 3 (M), Immunofluorescence analysis of p-ERK1/2 or EGR-1 protein expression and its localization in LE cells. Panel 4 (N), Knockdown of ERK1/2 or EGR-1 genes using SMARTpool siRNA resulted in 80% decrease in their respective protein level after 96 h based on Western blot analysis. O, Effects of ERK1/2 siRNA and (P) EGR-1 siRNA on the inhibitory effects of IFNT on PGT-mediated release of PGF2α in LE cells. *, CONT vs. IFNT, P < 0.05. **, IFNT vs. IFNT+ERK-siRNA or IFNT+EGR-1-siRNA, P < 0.05. All numerical values are expressed in mean ± sem of three (n = 3) independent experiments.
IFNT cross talk with EGFR through SRC and inhibited release of PGF2α through JAK-SRC-EGFR-RAS-RAF signaling pathways in LE cells
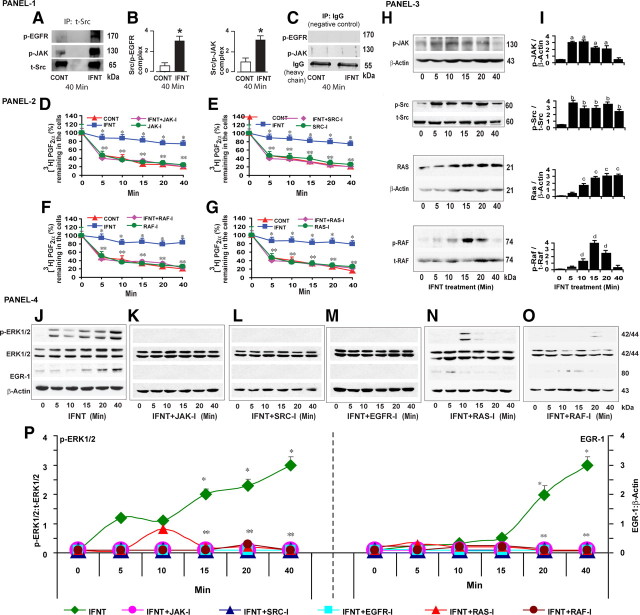
The signaling pathways through which IFNT interacts with EGFR in ruminant endometrium is not known. Recent studies have documented that c-SRC kinase is a major intracellular adopter protein that mediates interaction between tyrosine kinase receptor with other tyrosine kinase or G protein receptors in a variety of cells (16). In classical IFNT signaling, binding of IFNT with its receptors IFNR1 and IFNR2 phosphorylates JAK protein intracellularly (13). Based on this available information, we hypothesized that IFNT cross talks with EGFR through JAK and c-SRC kinases intracellularly in LE cells. We immunoprecipitated c-SRC protein and determined its interaction with JAK and EGFR proteins by Western blot. Results (Fig. 3, A–C) indicated that IFNT transactivated/phosphorylated (P < 0.05) EGFR protein through the JAK /SRC protein complex within 40 min in LE cells. Inhibition of JAK and SRC pathways reversed (P < 0.05) the inhibitory effects of IFNT on PGT-mediated 80% release of PGF2α from the LE cells (Fig. 3, D and E). Next, we determined whether IFNT signaling was routed through RAS and RAF pathways between EGFR and ERK1/2. Results indicated that inhibition of RAS and RAF pathways reversed (P < 0.05) the inhibitory effects of IFNT on PGT-mediated 80% release of PGF2α from the LE cells (Fig. 3, F and G). Inhibition of JAK, SRC, RAS, and RAF pathways did not show any nonspecific effect on PGT-mediated release of PGF2α in the absence of IFNT. Having established this functional data, we next sought to determine the effects of IFNT on activation of JAK, SRC, RAS, and RAF proteins by Western blot. Results (Fig. 3, H and I) indicated that IFNT phosphorylated/activated (P < 0.05) JAK, SRC, and RAF proteins between 10 and 20 min and RAS protein between 10 and 40 min in LE cells. This temporal activation of JAK, SRC, RAS, or RAF proteins was correlated (r = +0.91) with inhibitory effects of IFNT on PGT-mediated release of PGF2α by the LE cells. Because ERK1/2 and EGR-1 are the downstream mediators of the IFNT-JAK-SRC-EGFR-RAS-RAF signaling module, we examined whether inhibition of JAK, SRC, RAS, or RAF pathways suppressed effects of IFNT on activation of ERK1/2 or EGR-1 protein in LE cells. Results (Fig. 3, J–P) indicated that inhibition of JAK, SRC, EGFR, RAS, or RAF suppressed (P < 0.05) effects of IFNT on activation of ERK1/2 and EGR-1 proteins in LE cells in a temporal pattern. These results together suggest that IFNT inhibits PGT-mediated release of PGF2α through novel JAK-SRC-EGFR-RAS-RAF-ERK1/2-EGR-1 signaling module in the ovine endometrial LE cells.
Fig. 3.
Panel 1 (A), Interactions among SRC, JAK, and EGFR proteins in LE cells. Total Src protein was immunoprecipitated (IP), and its interaction with JAK or EGFR proteins was analyzed by Western blot. CONT, Control. B, Densitometry was determined by Alpha Imager, and relative expressions were expressed based on the integrated density values. *, CONT vs. IFNT, P < 0.05. C, As negative control IgG was immunoprecipitated, and interaction with JAK or EGFR proteins was examined. Panel 2 (D–G), Effects of JAK, SRC, RAS, and RAF pathways on inhibitory effects of IFNT on PGT-mediated release of PGF2α in LE cells. Inhibitors for JAK (AG490, 50 μm), SRC (PP2, 10 μm), RAS (Manumycin A, 10 μm), and RAF (Raf kinase inhibitor-1, 10 μm) were indicated by JAK-I, SRC-I, RAS-I, and RAF-I. *, CONT vs. IFNT, P < 0.05. **, IFNT vs. IFNT+JAK-I, IFNT+SRC-I, IFNT+RAS-I or IFNT+RAF-I, P < 0.05. Panel 3 (H), Effects of IFNT on phosphorylation and activation of JAK, SRC, RAS, and RAF proteins analyzed by Western blot. I, Densitometry was determined by Alpha Imager, and relative expressions were expressed based on the integrated density values. Effects of IFNT on (a) p-JAK protein at 0 vs. 5–20 min, (b) on p-Src protein at 0 vs. 5–40 min, (c) on RAS protein at 0 vs. 10–40 min, and (d) Raf protein 0 vs. 10–20 min, P < 0.05. Panel 4 (J–O), Effects of JAK, SRC, RAS, and RAF pathways on the inhibitory effects of IFNT on phosphorylation/activation of ERK1/2 and EGR-1 proteins in LE cells, analyzed by Western blot. P, Densitometry was determined by Alpha Imager, and relative expressions were expressed based on the integrated density values. *, Effects of IFNT on phosphorylation/activation of ERK1/2 or EGR-1 protein at 0 vs. 15–40 min, P < 0.05. **, IFNT vs. IFNT+JAK-I, IFNT+SRC-I, IFNT+RAS-I, or IFNT+RAF-I on phosphorylation/activation of ERK1/2 or EGR-1 protein at 0 vs. 15–40 min, P < 0.05. All numerical values are expressed in mean + sem of three (n = 3) independent experiments.
IFNT altered phosphorylation of PGT protein through ERK1/2 and EGR-1 pathways in LE cells
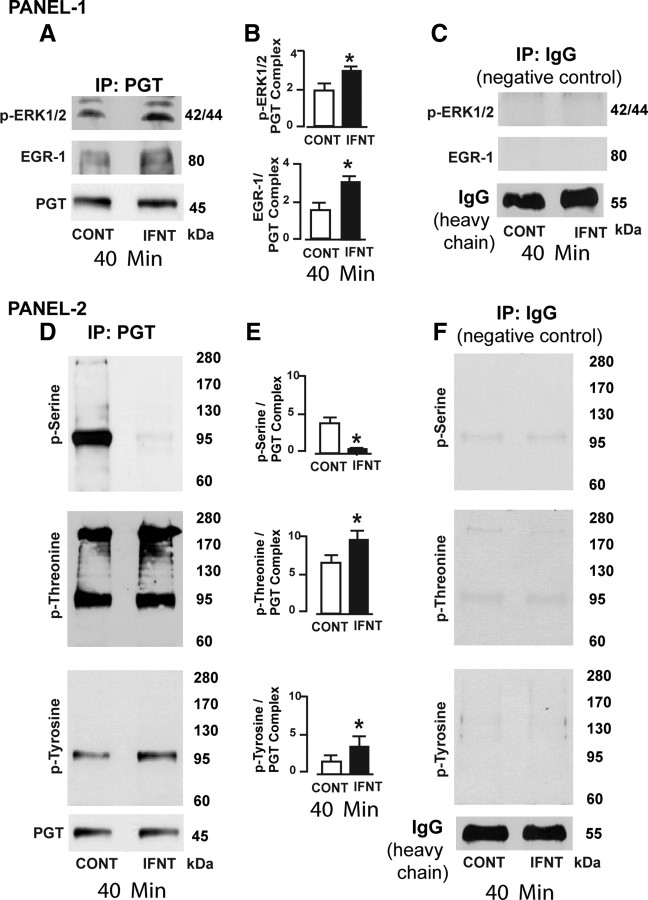
Our data (Figs. 2 and 3) indicated that ERK1/2 and EGR-1 proteins are the major downstream mediators through which IFNT inhibited PGT-mediated 80% release of PGF2α by the LE cells. This exciting finding further directed us to investigate whether ERK1/2 or EGR-1 protein directly interacted with PGT protein. We immunoprecipitated PGT protein and determined its interactions with pERK1/2 and EGR-1 proteins by Western blot. Results (Fig. 4, A–C) indicated that IFNT increased (P < 0.05) interactions among PGT, ERK1/2, and EGR-1 proteins within 40 min in LE cells. It is well characterized that ERK1 and ERK2 are phosphorylated at Thr202/Tyr204 and Thr185/Tyr187, respectively (17). EGR-1 is one of the important downstream mediators of the ERK1/2 pathway (18). In the present study, we found that IFNT did not decrease expression of PGT protein (Fig. 1) and suggested that PGT protein could be phosphorylated by ERK1/2 or EGR-1. We have previously reported that serine, threonine, and tyrosine phosphorylation sites for MAPK and EGFR are present in the extracellular and intracellular loops and transmembrane domains of ovine PGT (5). Therefore, we hypothesized that IFNT could alter phosphorylation of PGT through ERK1/2 or EGR-1. To test this hypothesis, we immunoprecipitated PGT protein and determined its interactions with phosphorylated serine, threonine, and tyrosine proteins by Western blot. Results (Fig. 4, D–F) indicated that IFNT increased (P < 0.05) interaction between PGT and phosphorylated threonine and tyrosine proteins and at the same time inhibited (P < 0.05) interaction between PGT and phosphorylated serine proteins. These results together suggest that IFNT could phosphorylate PGT protein at threonine and tyrosine residues and dephosphorylate PGT protein at serine residues through pERK1/2 and EGR-1 protein complex and thereby inhibit PGT-mediated release of PGF2α in the ovine endometrial LE cells.
Fig. 4.
IFNT phosphorylate of PGT in LE cells. Panel 1 (A), Interactions among PGT, p-ERK1/2, and EGR-1 proteins in LE cells. PGT protein was immunoprecipitated (IP), and its interaction with pERK1/2 or EGR protein was determined by Western blot. CONT, Control. B, Densitometry was determined by Alpha Imager, and relative expressions and interactions were expressed based on the integrated density values. *, CONT vs. IFNT, P < 0.05. C, As negative control IgG was immunoprecipitated and interaction with p-ERK or EGR-1 proteins was examined by Western blot. Panel 2 (D), Interactions among PGT, p-serine, p-threonine, and p-tyrosine proteins in LE cells. PGT protein was immunoprecipitated, and its phosphorylation status at p-serine, p-threonine, or p-tyrosine residues was determined by Western blot. E, Densitometry was determined by Alpha Imager, and relative expressions and interactions were expressed based on the integrated density values. *, CONT vs. IFNT, P < 0.05. F, As negative control IgG was immunoprecipitated, and interaction with p-serine, p-threonine, or p-tyrosine proteins was examined by Western blot. All numerical values are expressed in mean ± sem of three (n = 3) independent experiments.
IFNT activated JAK-SRC-EGFR-ERK1/2-EGR-1 signaling modules in ovine endometrium in vivo
We then examined this novel IFNT signaling in ovine endometrium in vivo. We employed the classical model used by Bazer and Spencer (19) to study P4-regulated and IFNT-stimulated genes in the ovine endometrium. Results (Fig. 5, A and B) indicated that IFNT phosphorylated or activated p-JAK, c-SRC, Ras, p-Raf, p-EGFR, p-ERK1/2, and EGR-1 proteins in endometrial luminal epithelia. Blockade of P4 action did not inhibit IFNT-stimulatory effects on activation of p-JAK, c-SRC, RAS, p-RAF, p-EGFR, and EGR-1 proteins but inhibited activation of p-ERK1/2 protein in endometrial luminal epithelia. IFNT did not modulate expression of PGT protein in endometrial luminal epithelia. These results together indicate that IFNT activates the JAK-SRC-EGFR-RAS-RAF-ERK1/2-EGR-1 signaling module without affecting PGT protein expression in ovine endometrium in vivo at the time of recognition of pregnancy and further confirm our in vitro findings.
Fig. 5.
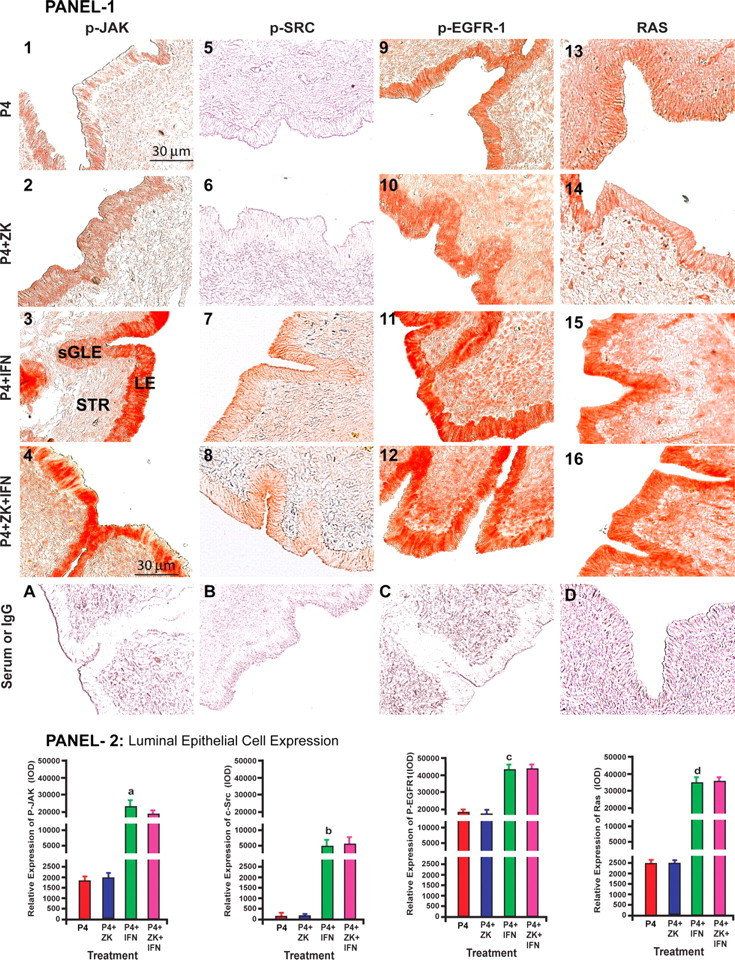
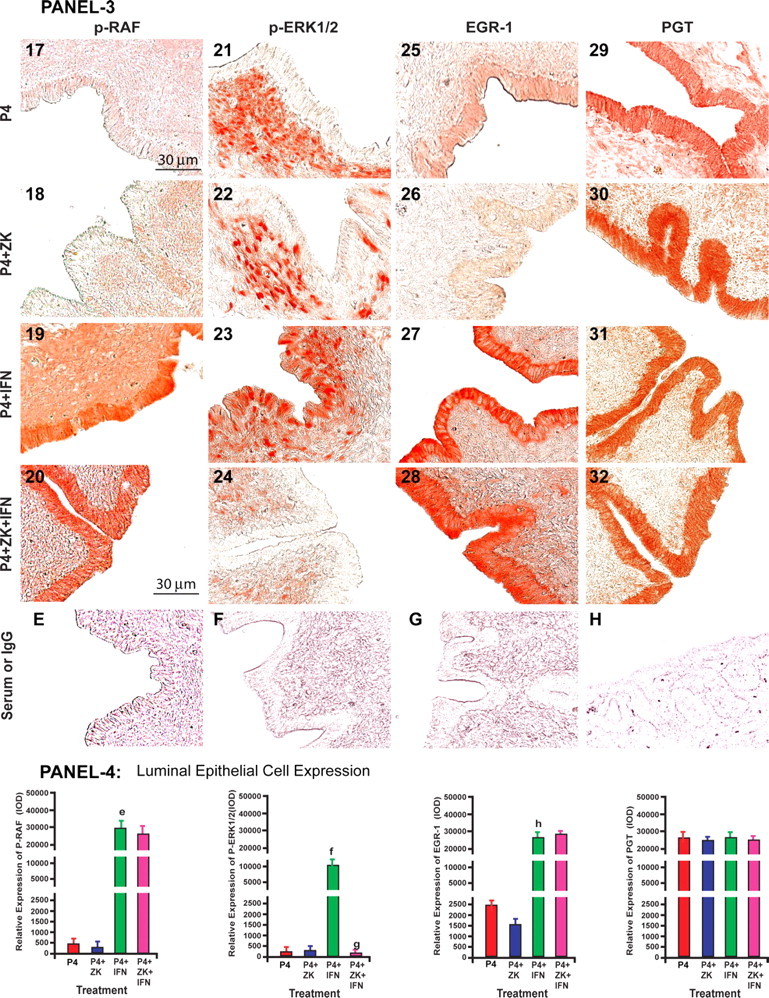
Panels 1 and 3: Effects of P4, P4+ZK, P4+IFNT, P4+ZK+IFNT on expression/activation of p-JAK (1–4), c-p-SRC (5–8), p-EGFR (9–12), RAS (13–16), p-RAF (17–20), p-ERK1/2 (17–20), EGR-1 (25–28), and PGT (29–32) proteins in LE. Serum or IgG (A–H). ZK, ZK 136,317; nuclear PGR antagonist, IFNT, and P4: Control. sGLE, and stromal cells (STR). Panels 2 and 4: Densitometry quantified using Image-Pro Plus. Effects of IFNT on of relative LE cell-specific expression of (a) p-JAK, (b) p-SRC, (c) p-EGFR, (d) RAS, (e) p-RAF, (f) p-ERK1/2, (h) EGR-1, and (g) p-ERK1/2, (P < 0.05), n = 4. Immunohistochemistry was performed using Vectastain Elite ABC kit, and representative photomicrographs at ×400 magnification are shown. Please see Materials and Methods for detailed description on treatment regimen.
IFNT precluded OTR-dependent as well as OTR-independent activation of PKC and Ca2+ signaling on PGT-mediated release of PGF2α in LE cells
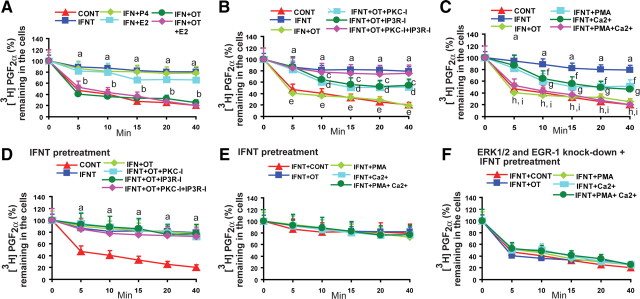
Finally, we determined whether P4, 17β-estradiol (E2), and OT modulated IFNT-inhibited and PGT-mediated release of PGF2α by the LE cells. Results (Fig. 6A) indicated that OT completely (80%) reversed (P < 0.05), E2 partially (20%) reversed (P < 0.05), P4 did not reverse, and OT and E2 did not additively reverse the effects of IFNT on PGT-mediated release of PGF2α by the LE cells. It suggests that OT is the sole mediator that mitigates actions of IFNT on PGT-mediated release of PGF2α in LE cells. It is well established that OT-OXTR signaling is mediated through PKC and inositol triphosphate (IP3)/Ca2+ pathways. To understand OXTR signaling interactions with the novel JAK-SRC-EGFR-RAS-RAF-ERK1/2-EGR-1 pathways in LE cells we inhibited PKC, IP3, or both pathways. Results indicated that inhibition of either the PKC or IP3 pathway partially suppressed (P < 0.05) the action of OT and inhibited 40% of PGT-mediated release of PGF2α. By contrast, inhibition of both PKC and IP3R completely suppressed the action of OT and inhibited (P < 0.05) 80% of PGT-mediated release of PGF2α (Fig. 6B). These results together suggest that activation of PKC and IP3/Ca2+ signaling is the critical mechanism that regulates PGT-mediated release of PGF2α in LE cells. Interestingly, OXTR is not the only candidate that activates PKC and Ca2+ pathways in endometrial LE cells. Therefore, we determined whether intracellular activation of PKC and Ca2+ independent of OXTR was able to release PGF2α. Results indicated that activation of either PKC or Ca2+ induced (P < 0.05) 40% of PGT-mediated release of PGF2α. Surprisingly, activation of both PKC and Ca2+ induced (P < 0.05) 80% PGT-mediated release of PGF2α (Fig. 6C). These results together confirm that intracellular activation of PKC and Ca2+, independent of OXTR, is also able to induce PGT-mediated release of PGF2α in LE cells.
Fig. 6.
A, Interactions among IFNT, P4, E2, and OT on PGT-mediated release of PGF2α in LE cells. B, Interactions among IFNT and OXTR signaling pathways on PGT-mediated release of PGF2α in LE cells. C, Interactions among IFNT and PKC and Ca2+ signaling pathways on PGT-mediated release of PGF2α in LE cells. Effects of pretreatment of IFNT on (D) OXTR signaling pathways and (E) PKC and Ca2+ signaling pathways on PGT-mediated release of PGF2α in LE cells. F, ERK1/2 and EGR-1 gene knockdown precluded effects of IFNT, OT, PKC, Ca2+ signaling on PGT-mediated release of PGF2α in LE cells. a, Control (CONT) vs. IFNT; b, IFNT vs. OT or OT+E2; c, IFNT vs. IFNT+OT+PKC-I; d, IFNT vs. IFNT+OT+IP3R-I; e, IFNT vs. IFNT+OT+PKC-I+IP3R-I; f, IFNT vs. IFNT+PMA; g, IFNT vs. IFNT+Ca2+; h, IFNT vs. IFNT+PMA+Ca2+; I, IFNT vs. IFNT+OT; P < 0.05. P4 (100 nm), E2 (10 nm), OT (100 nm), PKC-I (GF109203, 10 μm), IPR3-I (2APB, 25 μm), PKC activator (PMA, 100 nm), and Ca2+ ionophore (A23187, 1 μm) were used. All numerical values are expressed in mean ± sem of three (n = 3) independent experiments.
Collectively, these results suggest that both OXTR-dependent and OXTR-independent activation of PKC and Ca2+ signaling needs to be precluded by IFNT to inhibit PGT-mediated release of PGF2α from the LE cells at the time of recognition of pregnancy. In sheep, IFNT is secreted by the conceptus as early as d 10–d 11, but OXTR-induced pulsatile release of PGF2α from the endometria is inhibited by IFNT on d 14–d 16 of pregnancy, indicating priming of endometrium by IFNT about 5–6 d. To recapitulate the in vivo condition in vitro, we pretreated LE cells with IFNT for 60 min to activate JAK-SRC-EGFR-RAS-RAF-ERK1/2-EGR-1 signaling module as we established in the previous experiments (Figs. 2–4) and activated PKC and Ca2+. Results (Fig. 6, D and E) indicated that pretreatment of IFNT precluded (P < 0.05) actions of PKC and Ca2+ on PGT function. Thus, OT/OXTR-dependent or -independent activation of PKC and Ca2+ was not able to reverse (P < 0.05) the inhibitory effects of IFNT on 80% PGT-mediated release of PGF2α by the LE cells. Moreover, our data (Fig. 6F) indicated that double knockdown of ERK1/2 and EGR-1 completely inhibited (P < 0.05) pretreatment effects of IFNT on PGT-mediated release of PGF2α by the LE cells and further confirm the integral role of ERK1/2 and EGR-1 in IFNT signaling. These results together suggest that IFNT precludes both OXTR-dependent and OXTR-independent action of PKC and Ca2+ on PGT through JAK-SRC-EGFR-RAS-RAF-ERK1/2-EGR-1 signaling module and thereby inhibits PGT-mediated release of PGF2α by the ovine endometrial LE cells.
Discussion
In deciphering novel IFNT signaling, we examined roles for PKA, PKC, and EGFR cell-signaling pathways on the effects of IFNT on PGT-mediated release of PGF2α. The results indicated that inhibition of PKA and PKC pathways did not reverse but, interestingly, inhibition of EGFR pathways reversed the effects of IFNT on PGT-mediated release of PGF2α. Analysis of EGFR downstream pathways indicated that inhibition of ERK1/2 pathways reversed the effects of IFNT on release of PGF2α but not inhibition of PI3K/AKT, P38MAPK, and JNK/SAPK pathways. We examined a set of transcriptional factors EGR-1, ETS1/2, SP1, SP3, and p-ELK downstream of ERK1/2 pathways and found that IFNT increased expression/activation of EGR-1 but not other proteins. Further, immuonprecipitation analysis indicated that IFNT transactivated EGFR through JAK/SRC, and activation of EGFR, in turn, triggered RAS-RAF-ERK1/2 pathways. Activation of novel JAK-SRC-EGFR-RAS-RAF-ERK1/2-EGR-1 signaling pathways phosphorylated PGT protein at tyrosine and serine residues and dephosphorylated PGT protein at serine residues and thereby inhibited PGT-mediated 80% release of PGF2α in the endometrial LE cells. Further, intrauterine infusion of IFNT stimulated activation of JAK, SRC, EGFR, RAS, RAF, ERK1/2, and EGR-1 proteins in endometrial luminal epithelia in vivo. Interestingly, IFNT did not affect the expression of PGT in endometrial luminal epithelia, which is in agreement with our previous report in cattle (20). Together, our in vitro and in vivo data indicate that IFNT inhibits 80% of PGT-mediated release of PGF2α through activation of novel JAK-SRC-EGFR-RAS-RAF-ERK1/2-EGR-1 signaling module in the ovine endometrial LE cells.
At the time of luteolysis, OT is released from the posterior pituitary in a pulsatile pattern, acts on OXTR in the endometrial LE cells, and releases pulses of PGF2α. The timing of release of OT-induced PGF2α pulses from the endometria is closely associated with release of OT pulses from the posterior pituitary (1, 8). IFNT suppresses transcription of OXTR gene in LE cells through well-established IRF2-ESR1-OXTR pathways (2, 3) but did not inhibit OT pulses from the posterior pituitary (8). IFNT inhibits OXTR-dependent activation of PKC and Ca2+-signaling pathways by suppressing OXTR in endometrial LE cells. Importantly, data from the present study suggest that both OXTR-dependent and OXTR-independent activation of PKC and Ca2+ induce PGT-mediated release of PGF2α from the LE cells. Interestingly, OT/OXTR is not the only candidate activates PKC and Ca2+ pathways in endometrial LE cells. PGF2α receptor (FP) and PGE2 receptor (EP1 and EP3) are potential activators of PKC and Ca2+ pathways that are expressed in endometrial epithelia and conceptus and can be activated by PGF2α and PGE2 at the time of establishment of pregnancy in sheep. In addition, integrin receptors that activate PKC and IP3/Ca2+ signaling are expressed in endometrial epithelia and conceptus at the time of establishment of pregnancy in sheep (21, 22). These results together suggest that IFNT should inhibit OXTR-independent activation of PKC and Ca2+ signaling in addition to suppression of OXTR to inhibit PGT-mediated pulsatile release of PGF2α completely from the endometrial LE cells. Our data indicate, for the first time, that IFNT totally precludes actions of PKC and Ca2+ on PGT function through novel SRC-EGFR-RAS-RAF-ERK1/2-EGR-1 signaling module and thereby inhibits 80% of PGT mediated release of PGF2α from the LE cells. It is now clearly evident that IFNT activates IRF-2-ESR-1-OXTR and JAK-SRC-EGFR-RAS-RAF-ERK1/2-EGR-1 complementary signaling modules to inhibit pulsatile release of PGF2α from the endometrial LE cells at the time of recognition of pregnancy in ruminants. One of the most interesting aspects is that IFNT inhibits only 80% of PGT-mediated but not 20% of simple diffusion-mediated release of PGF2α from the LE cells, and this explains increased basal release of PGF2α in pregnant compared with nonpregnant sheep. Based on our results and available information, we proposed novel molecular mechanisms through which IFNT acts on endometrial LE cells and inhibits PGT-mediated release of PGF2α (Fig. 7).
Fig. 7.
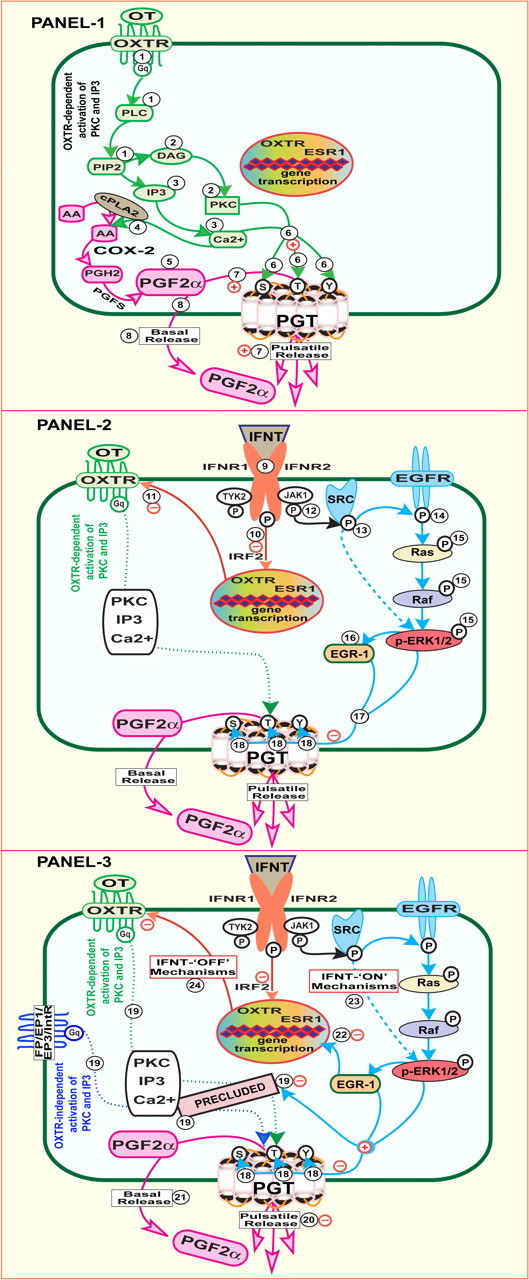
IFNT regulates PGF2α release from the ovine endometrial epithelial cells through PGT-mediated mechanisms by activating multiple signaling pathways. Panel 1, At the time of luteolysis, (1 ) OT binds with its receptor (OXTR) which coupled to Gq protein and activates phospholipase C (PLC) that results in generation of two second messengers (2 ) diacylglycerol that activates PKC and (3 ) IP3 which liberates intracellular calcium (Ca2+). (4 ) Increased intracellular concentration of PKC and Ca2+ releases arachidonic acid from the phospholipids and thus (5 ) increases intracellular concentration of PGF2α through phospholipase A2 (cPLA2), cyclooxygenase-2 (COX-2) and prostaglandin F synthase (PGFS), and (6 ) phosphorylates or dephosphorylates PGT protein at specific serine, threonine, and tyrosine residues. (7 ) Activated PGT facilitates transport (80%) of PGF2α as pulsatile release through PGT-dependent mechanism. In addition, (8 ) 20% of PGF2α can be transported as basal release through PGT-independent mechanism. Panel 2, At the time of establishment of pregnancy, (9 ) IFNT interacts with its receptors IFNR1 and IFNR2 and (10 ) induces expression of IRF-2, and thereby (11 ) suppresses transcription of ESR1 and OXTR genes. In addition to IRF2-ESR-1-OXTR module, (12 ) IFNT phosphorylates JAK protein through IFNR1 and IFNR2. Activated JAK (13 ) phosphorylates SRC protein which in turn (14 ) phosphorylates EGFR protein. Activated EGFR (15 ) phosphorylates ERK1/2 proteins through RAS and RAF pathways. Activated ERK1/2 (16 ) increases the expression of EGR-1 protein. (17 ) Activated ERK1/2 along with EGR-1 (18 ) phosphorylates PGT protein at tyrosine and threonine residues and dephosphorylates PGT protein at serine residues. Panel 3, IFNT through novel JAK-SRC-EGFR-RAS-RAF-ERK1/2-EGR-1 signaling module alters the (18 ) functional state of PGT and (19 ) precludes OXTR-dependent and OXTR-independent actions of PKC, IP3, and Ca2+ on PGT function. Thus, IFNT (20 ) inhibits PGT-dependent pulsatile release of PGF2α and (21 ) does not inhibit PGT-independent basal release of PGF2α, and this explains increased basal release of PGF2α in pregnant compared with nonpregnant sheep. It is known that IFNT inhibits transcription of OXTR and ESR1 genes through IRF2 by precluding interaction between ESR1 and SP1 proteins. EGR-1 and SP1 proteins are competing for the same GC-rich element in the given promoters. We propose that (22 ) increased EGR-1 protein might compete with SP1 protein and act as potential transcriptional suppressor for ESR1 and OXTR genes. (Note: This mechanism was not confirmed in the present investigation.) Together, (23 ) IFNT-IFNR-JAK-SRC-EGFR-RAS-RAF-ERK1/2-EGR-1 signaling module (IFNT-ON mechanisms) and (24 ) IFNT-IFNR-IRF-2-ESR-1-OXTR signaling module (IFNT-OFF mechanisms) are complementary signaling mechanisms required for IFNT to inhibit OXTR-dependent and OXTR-independent activation of PKC, IP3, and Ca2+ and to inhibit pulsatile release of PGF2α by the endometrial LE cells at the time of establishment of pregnancy in ruminants.
PGT belongs to the organic anion transporter polypeptide family that shares common structural features that include: 12-transmembrane domains, multiple glycosylation sites, multiple phosphorylation sites in the intracellular loops, extracellular loops, and transmembrane domains, presence of clusters of cysteine zinc-finger motifs in the extracellular loops, and presence of predominantly positive charged amino acid residues (23). The phosphorylated state of several serine, threonine, and tyrosine residues in response to PKC (24, 25), MAPK (26, 27), and AKT (28) regulates phosphorylation, cell surface expression, internalization, stability, maximum affinity (Km), and maximum transport velocity (Vmax) of organic anion transporter polypeptides (23, 29). We have reported in our previous study that PKA and PKC pathways are involved in PGT-mediated influx/uptake transport, ERK1/2 pathways are involved in PGT-mediated efflux/release transport, and JNK/SAPK pathways are involved in PGT-mediated efflux and influx in LE cells (5). The results of the present study indicate that IFNT activates only ERK1/2 but not other pathways, and IFNT phosphorylates PGT protein at tyrosine and threonine and dephosphorylates PGT protein at serine residues in LE cells. These results together suggest that the functional activity (release or uptake) of PGT can be modulated by its interaction with specific intracellular cell-signaling pathways. At this time, molecular mechanisms through which ERK1/2 and EGR-1 interact with PGT and which specific serine, threonine, and tyrosine residues of PGT are phosphorylated or dephosphorylated by IFNT-JAK-SRC-EGFR-RAS-RAF-ERK1/2-EGR-1signaling module is not clear. Indeed, unraveling this mechanism is our future focus.
As detailed in the introduction, cell-signaling pathways through which IFNT stimulates epithelial cell- specific genes in ruminant endometrium is not known. Results of the present study add new information on action of IFNT in endometrial LE cells. This novel IFNT-JAK-SRC-EGFR-RAS-RAF-ERK1/2-EGR-1 signaling module needs to be explored in detail to understand molecular and cellular mechanisms through which IFNT stimulates several genes in endometrial LE cells that are required for conceptus elongation and development. It has been reported that IFNT precluded binding of ESR1/SP1 proteins with guanine-cytosine (GC)-rich regions of OXTR promoter and thereby indirectly inhibited transcription of OXTR genes through ESR/SP1 complex (30). The present investigation finds that IFNT increases EGR-1 protein in LE cells in vitro and in vivo. It is well established that EGR-1 and SP1 are competing for the same GC-rich element in the given promoters under certain physiological conditions (31, 32, 33). Further, EGR-1 acts as tissue-specific transcriptional suppressor or activator of various genes under different physiological and pathological conditions (31, 32, 33). Given the facts, IFNT could suppress ESR1, OXTR, or preclude binding of ESR/SP1 with OXTR promoter through increased EGR-1 by activating JAK-SRC-EGFR-RAS-RAF-ERK1/2-EGR-1 signaling module in endometrial LE cells. However, this alternative complementary mechanism needs to be examined by future studies.
In conclusion, the new findings of the present investigation are 2-fold: 1) IFNT activates novel JAK-SRC-EGFR-RAS-RAF-ERK1/2-EGR-1 signaling modules in endometrial LE cells and 2) IFNT regulates PGT-mediated release of PGF2α from the endometrial LE cells through these novel cell-signaling pathways. Our results provide new insight into molecular mechanisms on actions of IFNT and release of PGF2α from the endometrial LE cells and thus open a new arena of research in IFNT signaling and PGT function. The novel signaling pathways identified in this study could emerge as potential targets for molecular therapy to improve reproductive efficiency in agriculturally important ruminants such as cattle and sheep. Information obtained on PGT function could be extrapolated to other mammalian species including human and to other physiological and pathological conditions in which prostaglandins play important roles.
Materials and Methods
Materials
The reagents used in this study were purchased from the following suppliers: Prestained protein markers and Bio-Rad assay reagents and standards (Bio-Rad Laboratories, Hercules, CA); [3H]PGF2α (PerkinElmer Life Sciences); Protran BA83 Nitrocellulose membrane (Whatman, Inc, Sanford, ME); Pierce ECL (Pierce Biotechnology, Rockford, IL); protease inhibitor cocktail tablets complete EDTA-free and PhosStop (Roche Applied Biosciences, Indianapolis, IN); antibiotic-antimycotic, and trypsin-EDTA, Alexa Fluor 594, and ProLong Gold antifade reagent (Invitrogen Life Technologies, Inc, Carlsbad, CA); Vectastain Elite ABC kit (Vector Laboratories, Inc, Burlingame, CA); Blue X-Ray film (Phenix Research Products, Hayward, CA); fetal bovine serum (HyClone Laboratories, Logan, UT); Lab-Tek II chamber slides (Nunc, Rochester, NY), and tissue culture dishes and plates (Corning, Inc, Corning, NY). ERK1/2 and EGR-1 small interfering RNA (siRNA), siGLORISC-free siRNA, and DharmaFect-1 were obtained from Dharmacon, Inc. (Lafayette, CO). Inhibitors for PKA (H-89), PKC (GF109203), EGFR (AG1478), ERK1/2 (U0126), P38MAPK (SB203580), JNK/SAPK (SP600125), PI3K (LY294002), JAK (AG490), Src (PP2), Ras (Manumycin A), and Raf (Raf kinase inhibitor 1) were purchased form EMD Biosciences (San Diego, CA). P4, E2, OT, phorbol-12-myristate 13-acetate (PMA) Ca2+ ionophore (A23187), IP3R inhibitor (2APB), and rabbit IgG were purchased from Sigma-Aldrich (St. Louis, MO). Details on antibody used are given in Table 1. All antibodies used in this study were purchased from Cell Signaling Technology (Danvers, MA) or Santa Cruz Biotechnology, Inc., (Santa Cruz, CA) except β-actin monoclonal antibody (Sigma-Aldrich, St. Louis, MO), goat antirabbit or antimouse IgG conjugated with horseradish peroxidase (Kirkegaard & Perry Laboratories, Gaithersburg, MD). The chemicals used were molecular biological grade from Fisher Scientific (Pittsburgh, PA) or Sigma-Aldrich.
Table 1.
List of antibodies used
| No. | Name | Source | Phosphorylation sites | Concentration | Catalog no. | Manufacturer |
|---|---|---|---|---|---|---|
| 1 | p-JAK1 | Rabbit polyclonal | Tyr1022/1023 | 1:1000 | 3331 | Cell Signaling |
| 2 | JAK1 | Rabbit monoclonal | 1:1000 | 3344 | Cell Signaling | |
| 3 | p-SRC 416 | Rabbit polyclonal | Tyr416 | 1:1000 | 2101 | Cell Signaling |
| 4 | SRC | Rabbit monoclonal | 1:1000 | 2109 | Cell Signaling | |
| 5 | p-β-Arrestin1 | Mouse monoclonal | Ser412 | 1:1000 | 2416 | Cell Signaling |
| 6 | p-EGFR | Goat polyclonal | Tyr1173 | 1:500 | sc-12351 | Santa Cruz |
| 7 | EGFR | Rabbit polyclonal | 1:500 | sc-03 | Santa Cruz | |
| 8 | RAS | Rabbit polyclonal | 1:1000 | 3965 | Cell Signaling | |
| 9 | p-c-RAF | Rabbit monoclonal | Ser338 | 1:1000 | 9427 | Cell Signaling |
| 10 | c-RAF | Rabbit polyclonal | 1:1000 | 9422 | Cell Signaling | |
| 11 | p-ERK1/2 | Rabbit Polyclonal | Thr202/Tyr204 | 1:1000 | 9101 | Cell Signaling |
| 12 | ERK1/2 | Rabbit polyclonal | 1:1000 | 9102 | Cell Signaling | |
| 13 | p-AKT | Rabbit polyclonal | Ser473 | 1:1000 | 9271 | Cell Signaling |
| 14 | AKT | Rabbit polyclonal | 1:1000 | 9272 | Cell Signaling | |
| 15 | p-JNK | Rabbit polyclonal | Thr183/Tyr185 | 1:1000 | 9251 | Cell Signaling |
| 16 | JNK | Rabbit monoclonal | 1:1000 | 9258 | Cell Signaling | |
| 17 | p-P38 | Rabbit polyclonal | Thr180/Tyr182 | 1:1000 | 9211 | Cell Signaling |
| 18 | P38 | Rabbit polyclonal | 1:1000 | 9251 | Cell Signaling | |
| 19 | EGR1 | Rabbit polyclonal | 1:1000 | 4152 | Cell Signaling | |
| 20 | SP1 | Rabbit polyclonal | 1:500 | sc-59 | Santa Cruz | |
| 21 | ETS-1/2 | Rabbit polyclonal | 1:500 | sc-112 | Santa Cruz | |
| 22 | p-ELK-1 | Rabbit polyclonal | Ser383 | 1:1000 | 9181 | Cell Signaling |
| 23 | ELK-1 | Rabbit polyclonal | 1:1000 | 9182 | Cell Signaling | |
| 24 | SP3 | Rabbit polyclonal | 1:500 | sc-644 | Santa Cruz | |
| 25 | β-Actin | Mouse monoclonal | 1:8000 | A2228 | Sigma-Aldrich | |
| 26 | p-Serine | Rabbit polyclonal | Ser | 1:1000 | 9601 | Cell Signaling |
| 27 | p-Threonine | Mouse monoclonal | Thr | 1:1000 | 9386 | Cell Signaling |
| 28 | p-Tyrosine | Mouse monoclonal | Tyr | 1:2000 | 9411 | Cell Signaling |
| 29 | PGT | Rabbit polyclonal | 1:1000 | 160200 | Cayman Chemicals |
Cell culture
Ovine immortalized endometrial LE cells (34) were cultured in DMEM/F12 medium containing 5% dextran charcoal-treated fetal bovine serum (DC-FBS) and 100 U penicillin/ml, 100 μg streptomycin/ml, and 2.5 μg amphotericin B/ml in humidified 5% CO2 and 95% air at 37 C. These cells expressed IFNT receptors IFNR1 and IFNR2 and responded to IFNT (13, 35).
Influx/uptake experiments
The cells were cultured in 24-well plates. The influx of PGF2α was determined as we described previously (4, 5). The LE cells were pretreated with or without IFNT [1 μg, 106 antiviral units (AVU)] for 60 min after which medium was replaced and cells were incubated with [3H]PGF2α (1.0 nm, well below the Km) for 0, 5, 10, 15, 20, or 40 min. At each time point, the cells were washed with ice-cold Hank’s Balanced Salt Solution (HBSS), harvested using trypsin-EDTA, and [3H]PGF2α uptake was determined using a β-scintillation counter (Beckman Coulter, Inc., Fullerton, CA). Uptake rates were calculated in percentage (%) as the amount of radiolabeled PGF2α effluxed into the cells. The radioactivity remaining in the cells at time 0 was considered as 0%. Data were expressed as the mean ± se of three experiments.
Efflux/release experiments
PGF2α efflux experiments in LE cells were performed as described (4, 5) with little modifications. The LE cells were cultured in 24-well plates, serum starved as described above, and incubated with [3H]PGF2α (1.0 nm, well below the Km) for 10 min (the time point when maximum influx was found in influx experiment). Then, the medium was replaced with fresh medium with or without IFNT (1μg = 106 AVU). This time point was considered as time zero, and cells were harvested at various time points 0, 5, 10, 15, 20, and 40 min. At each time point, the cells were washed with ice-cold HBSS, harvested using trypsin-EDTA, and [3H]PGF2α uptake was determined using a β-scintillation counter (Beckman Coulter, Inc., Fullerton, CA). Efflux rates were calculated in percentage (%) as the amount of [3H]PGF2α effluxed from the cells. The radioactivity remaining in the cells at time zero was considered as 100%. Data were expressed as the mean ± se of three experiments.
PGF2α release experiments with IFNT and inhibitors for several cell-signaling pathways were performed as follows: The LE cells were cultured in 24-well plates, serum starved as described above, and incubated with [3H]PGF2α for 10 min. Then, the medium was replaced with fresh medium with or without IFNT (1 μg = 106 AVU) in the presence or absence of inhibitors for PKA (H-89, 50 nm), PKC (GF109203, 10 μm), EGFR (AG1478, 15 μm), ERK1/2 (U0126, 10 μm), P38MAPK (SB203580, 10 μm), JNK/SAPK (SP600125, 10 μm), PI3K (LY294002, 50 μm), JAK (AG490, 50 μm), Src (PP2, 10 μm), Ras (Manumycin A, 10 μm), and Raf (Raf kinase inhibitor 1, 10 μm). This time point was considered as ‘0’, and cells were harvested at various time points 0, 5, 10, 15, 20 and 40 min.
PGF2α release experiments with IFNT and P4, E2, and OT were performed as follows: The LE cells were cultured in 24-well plates, serum starved as described above, and incubated with [3H]PGF2α for 10 min. Then, the medium was replaced with fresh medium with or without IFNT (1μg = 106 AVU) in the presence or absence of P4 (100 nm), E2 (10 nm), OT (100 nm), or E2 + OT, PKC-I (GF109203, 10 μm), IP3-I (2APB, 25 μm), PKC-I + IP3-I, PKC activator (PMA, 100 nm), or Ca2+ ionophore (A23187, 1 μm), or PKC activator + Ca2+ ionophore. This time point was considered as ‘0’ and cells were harvested at various time points: 0, 5, 10, 15, 20, and 40 min.
PGF2α release experiments with IFNT pretreatment and OTR pathways were performed as follows: The LE cells were cultured in 24-well plates, serum starved as described above and incubated with [3H]PGF2α for 10 min. The cells were treated with IFNT for 60 min and then OT (100 nm) PKC activator (PMA, 100 nm), or Ca2+ ionophore (A23187, 1 μm), or PKC activator + Ca2+ ionophore were added. This time point was considered as ‘0’ and cells were harvested at various time points: 0, 5, 10, 15, 20, and 40 min.
In all PGF2α release experiments, at each time point, the cells were washed with ice-cold HBSS, harvested using trypsin-EDTA, and [3H]PGF2α uptake was determined using a β-scintillation counter (Beckman Coulter, Inc.). Efflux rates were calculated in percentage (%) as the amount of [3H] PGF2α effluxed from the cells. The radioactivity remaining in the cells at time ‘0’ was considered as 100%. Data were expressed as the mean ± se of three experiments.
ERK1/2 and EGR-1 siRNA
The ERK1/2 or EGR-1 genes were silenced to confirm its role in IFNT signaling on PGT-mediated release of PGF2α in LE cells. The endometrial LE cells were cultured in antibiotic-free DMEM/F12 medium with 10% DC-FBS in 24-well tissue culture plates. At 70–80% confluency, cells were used for knockdown experiments, and ERK1/2 or EGR-1 genes were silenced using respective SMARTpool siRNA duplex delivered by DharmaFect-1 per manufacturers’ instructions. As internal controls, siGLO RISC-Free siRNA or mock siRNA were used. According to the manufacturer’s instructions, SMARTpool siRNA consisted of at least four individual siRNA duplexes targeted against a specific gene and designed using a bioinformatics technology known as SMARTselection. This resulted in the generation of siRNAs more than 97% of the time, and the targeted message level was reduced by more than 70% within 24 h after transfection. We preferred SMARTpool siRNA compared to use more than one siRNA duplex from different regions of the gene of interest to avoid nonspecific indirect effects of single siRNA duplex to a single region of the target gene. Briefly, siRNA duplexes (100 nm/well) and DharmaFect-1 (1 μl/well) were diluted in 50 μl antibiotic and serum-free DMEM/F12 medium separately, mixed gently, and incubated for 5 min at room temperature. Afterward, PGT siRNA and DharmaFect-1 were mixed (total volume 100 μl) and incubated at room temperature for 20 min. Then, 100 μl siRNA: DharmaFect-1 complex were added to each well in a total volume of 400 μl/well antibiotic-free DMEM/F12 medium with 10% DC-FBS. After 24 h, the medium was replaced with fresh DMEM/F12 with 10% DC-FBS and incubated for a second 24-h period. PGF2α release/efflux experiments were performed 48 h after transfection using [3H]PGF2α as described above. Fluorescence-labeled siGLORISC-free siRNA was transfected separately, and transfection efficiency was estimated using a fluorescence microscope. A transfection efficiency of greater than 80% was considered ideal for these experiments. Knockdown of ERK1/2 or EGR-1 resulted in 80% decrease at protein level after 96 h based on Western blot analysis.
Protein extraction and immunoblotting
The effects of IFNT on expression/activation various signaling pathway proteins and their association with PGF2α transport in endometrial LE cells were determined using Western blot. The cells were cultured in 100-mm tissue culture dishes and serum starved. The cells were pretreated with or without inhibitors for EGFR (AG1478, 15 μm), JAK (AG490, 50 μm), Src (PP2, 10 μm), Ras (Manumycin A, 10 μm), and Raf (Raf kinase inhibitor 1, 10 μm) for 60 min and then treated with IFNT (1 μg = 106 AVU) for 0, 5, 10, 15, 20, or 40 min. Total protein was isolated and Western blot was performed as we described previously (5). Briefly, the cells were harvested using 1% Trypsin-EDTA and pelleted. The cell lysates were sonicated in sonication buffer, which consisted of 20 mm Tris-HCl, 0.5 mm EDTA, 100 μm diethyldithiocarbamic, 1% Tween, 1 mm phenylmethylsulfonyl fluoride, and protease inhibitor cocktail tablets: complete EDTA-free (1 tablet/50 ml) and PhosStop (1 tablet/10 ml). Sonication was performed using a Microson ultrasonic cell disruptor (Microsonix, Inc., Farmingdale, NY). Protein concentration was determined using the Bradford method and a Bio-Rad Protein Assay kit. Protein samples (75 μg) were resolved using 7.5%, 10%, or 12.5% SDS-PAGE. Chemiluminescent substrate was applied according to the manufacturer’s instructions (Pierce Biotechnology). The blots were exposed to Blue x-ray film, and densitometry of autoradiograms was performed using an Alpha Imager (Alpha Innotech Corp., San Leandro, CA).
Immunoprecipitation
The endometrial LE cells were cultured, treated, and harvested, after which total cell lysates were prepared as described above. Total cell lysate (1 mg) was precleared by incubating with appropriate preclearing matrix (Santa Cruz Biotechnology) for 30 min at 4 C. The precleared cell lysate was incubated with primary antibody (rabbit polyclonal PGT or t-Src, each at 1 μg) overnight at 4 C, and then further incubated with immunoprecipitation matrix-ExactraCruz (Santa Cruz Biotechnology) overnight at 4 C. Protein-antibody complexes were precipitated using protocols provided by Santa Cruz Biotechnology and/or Cell Signaling Technology as we described previously (36). As negative control, cell lysate was incubated with rabbit IgG (1 μg). Interactions with interested proteins as detailed in Results were determined by Western blot. Densitometry of autoradiograms was performed using an Alpha Imager.
Immunofluorescence
The endometrial LE cells were seeded at 50,000 cells per well on Lab-Tek II chambered slides and cultured as described above (5, 36). At 70–80% confluency the cells were treated with IFNT (1 μg = 106 AVU) for 40 min as described above. The procedure given by Cell Signaling Technology was followed with minor modifications. Cells were rinsed in PBS, fixed in 1% paraformaldehyde for 15 min at room temperature, and permeabilized for 10 min in 100% methanol at −20 C. Cells were blocked for 1 h in 10% normal serum from the same species in which secondary antibody was developed and then incubated overnight with primary antibodies (EGFR or EGR-1) at concentrations of 1:50 dilution. For the negative control, serum or IgG from respective species with reference to the primary antibody at the respective dilution was used. After washing in 0.2 m PBS/0.3% Tween, cells were incubated with Alexa Fluor 594-conjugated secondary antibodies for 1 h. Cells were washed and mounted with ProLong Gold antifade reagent. Images were visualized by using digital imaging and an image analysis workstation consisting of a Zeiss Axioplan 2 Research Microscope interfaced with a Zeiss Axiocam HR high-resolution color CCD camera with Zeiss Axiovision (Carl Zeiss, Thornwood, NY).
In vivo study
This study was performed as described by Bazer and Spencer (15) previously. Cyclic ewes (n =20) were checked daily for estrus. On d 5 of the estrous cycle the ewes were ovariectomized, and both uterine horns were fitted with indwelling uterine catheters. Ewes were then assigned randomly (n =5 per treatment) to receive the following treatment: group 1 (P4) ewes received P4 (50 mg/24 h) by im injections from d 5–d 16 and control serum protein (50 mg/horn/12 h, total 200 μg/24 h) by intrauterine injections from d 11–d 16. Group 2 (P4+ZK) ewes received P4 (50 mg/24 h) and nuclear PGR antagonist ZK 136,317 (75 mg/24 h, Schering AG, Berlin, Germany) by im injection from d 5–d 16 and control serum protein as in group 1 from d 11–d 16. Group 3 (P4+IFNT) ewes received P4 as in group 1 and IFNT (5 × 107 antiviral units/horn/12 h, total 2 × 107 AVU) by intrauterine injections from d 11–d 16. Group 4 ewes received P4 and ZK as in group 2 and IFNτ as in group 3. All ewes were hysterectomized on d 17 and several sections (0.5 cm) from the midportion of uterine horn ipsilateral to the corpus luteum were collected.
Immunohistochemistry
Endometrial tissue sections were fixed in 4% buffered paraformaldehyde saline for 4 h at 4 C and processed using standard procedures (5, 36). Paraffin sections (5 μm) were used for immunohistochemical localization of proteins involved in IFNT signaling using a Vectastain Elite ABC kit as previously we described (5, 36) and according to the manufacturer’s protocols. The tissue sections were incubated at 4 C overnight with specific antibodies at the concentrations recommended by manufacturers. Then, tissue sections were further incubated with the secondary antibody (biotinylated IgG) for 45 min at room temperature. For the negative control, serum or IgG from respective species with reference to the primary antibody at the respective dilution was used. Digital images were captured using a Zeiss Axioplan 2 Research Microscope (Carl Zeiss, Thornwood, NY) with an Axiocam HR digital camera. The intensity of staining for each protein was quantified using Image-pro Plus as we described previously (37) according to the manufacturer’s (Media Cybernetics, Inc., Bethesda, MD) instructions. We preferred immunohistochemistry followed by densitometry compared with Western blot because it will provide details on spatial expression of a specific protein in LE, sGLE, and stromal cells of endometrium whereas Western blot only provides information on total steady-state expression levels of a particular protein in the endometrium.
Statistical analyses
Statistical analyses were performed using general linear models of Statistical Analysis System (SAS, Cary, NC). Effects of treatment (IFNT and/or inhibitors), time, and treatment x time interactions on PGF2α transport and expression of various proteins were analyzed by repeated-measures multivariate ANOVA. Effect of IFNT on protein and protein interaction was determined by one-way ANOVA and followed by Tukey-Kramer honestly significant difference test. Simple linear correlation was used to determine association between treatment and effects. Statistical significance was considered as P < 0.05. Numerical data are expressed as mean ± sem. The statistical model accounted for sources of variation including treatments, replicates, and ewes as appropriate.
Acknowledgments
We thank Dr. Fuller W. Bazer and Dr. Thomas E. Spencer (Department of Animal Sciences, Texas A&M University, College Station, TX) for providing immortalized ovine endometrial LE cells, recombinant ovine IFNT, and subset of uterine tissues sections from their sharing recourses as generous gifts to complete the study. We also thank Dr. Robert C. Burghardt (Department of Veterinary Integrative Biosciences, Texas A&M University, College Station, TX) and Image Analysis Laboratory at College of Veterinary Medicine and Biomedical Sciences for the technical service/advise provided.
Footnotes
This research project was supported by National Research Initiative Competitive Grant 2008-35203-19101 from the US Department of Agriculture National Institute of Food and Agriculture (to J.A.A.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 20, 2010
S.K.B. and J.L. contributed equally to this work.
Abbreviations: AVU, Antiviral units; DC-FBS, dextran charcoal-treated fetal bovine serum; E2, 17β-estradiol; EGFR, epidermal growth factor receptor; EGR, early growth response; ESR1, estrogen receptor α; GC, guanine-cytosine; HBSS, Hank’s balanced salt solution; IFNT, interferon tau; ISGs, IFN-stimulated genes; IP3, inositol triphosphate; IRF, IFN-regulatory factor; JAK, Janus kinase; LE cells, luminal epithelial cells; OT, oxytocin; OXTR, oxytocin receptor; P4, progesterone; PGF2α, prostaglandin F2α; PGR, P4 receptor; PGT, prostaglandin transporter; PI3K, phosphatidylinositol-3-kinase; PKA, protein kinase A; PKC, protein kinase C; PMA, phorbol-12-myristate 13-acetate; sGLE, superficial glandular epithelial; siRNA, small interfering RNA; SRC, SRC kinase; STAT, signal transducer and activator of transcription.
References
- 1.McCracken JA, Custer EE, Lamsa JC1999. Luteolysis: a neuroendocrine-mediated event. Physiol Rev 79:263–323 [DOI] [PubMed] [Google Scholar]
- 2.Spencer TE, Burghardt RC, Johnson GA, Bazer FW2004. Conceptus signals for establishment and maintenance of pregnancy. Anim Reprod Sci 82–83:537–550 [DOI] [PubMed]
- 3.Spencer TE, Johnson GA, Bazer FW, Burghardt RC2007. Fetal-maternal interactions during the establishment of pregnancy in ruminants. Soc Reprod Fertil (Suppl 64):379–396 [DOI] [PubMed]
- 4.Banu SK, Arosh JA, Chapdelaine P, Fortier MA2003. Molecular cloning and spatio-temporal expression of the prostaglandin transporter: a basis for the action of prostaglandins in the bovine reproductive system. Proc Natl Acad Sci USA 100:11747–11752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banu SK, Lee J, Satterfield MC, Spencer TE, Bazer FW, Arosh JA2008. Molecular cloning and characterization of prostaglandin transporter in ovine endometrium: role of mitogen activated protein kinase pathways in release of prostaglandin F2 α. Endocrinology 149:219–231 [DOI] [PubMed] [Google Scholar]
- 6.Lee J, McCracken JA, Banu SK, Rodriguez R, Nithy TK, Arosh JA2010. Transport of prostaglandin F2α pulses from the uterus to the ovary at the time of luteolysis in ruminants is regulated by prostaglandin transporter-mediated mechanisms. Endocrinology 151:3326–3335 [DOI] [PubMed] [Google Scholar]
- 7.Zarco L, Stabenfeldt GH, Basu S, Bradford GE, Kindahl H1988. Modification of prostaglandin F-2 α synthesis and release in the ewe during the initial establishment of pregnancy. J Reprod Fertil 83:527–536 [DOI] [PubMed] [Google Scholar]
- 8.Hooper SB, Watkins WB, Thorburn GD1986. Oxytocin, oxytocin-associated neurophysin, and prostaglandin F2 α concentrations in the utero-ovarian vein of pregnant and nonpregnant sheep. Endocrinology 119:2590–2597 [DOI] [PubMed] [Google Scholar]
- 9.Charpigny G, Reinaud P, Tamby JP, Créminon C, Martal J, Maclouf J, Guillomot M1997. Expression of cyclooxygenase-1 and -2 in ovine endometrium during the estrous cycle and early pregnancy. Endocrinology 138:2163–2171 [DOI] [PubMed] [Google Scholar]
- 10.Kim S, Choi Y, Spencer TE, Bazer FW2003. Effects of the estrous cycle, pregnancy and interferon τ on expression of cyclooxygenase two (COX-2) in ovine endometrium. Reprod Biol Endocrinol 1:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi Y, Johnson GA, Burghardt RC, Berghman LR, Joyce MM, Taylor KM, Stewart MD, Bazer FW, Spencer TE2001. Interferon regulatory factor-two restricts expression of interferon-stimulated genes to the endometrial stroma and glandular epithelium of the ovine uterus. Biol Reprod 65:1038–1049 [DOI] [PubMed] [Google Scholar]
- 12.Wang SZ, Roberts RM2004. Interaction of stress-activated protein kinase-interacting protein-1 with the interferon receptor subunit IFNAR2 in uterine endometrium. Endocrinology 145:5820–5831 [DOI] [PubMed] [Google Scholar]
- 13.Stewart MD, Johnson GA, Vyhlidal CA, Burghardt RC, Safe SH, Yu-Lee LY, Bazer FW, Spencer TE2001. Interferon-τ activates multiple signal transducer and activator of transcription proteins and has complex effects on interferon-responsive gene transcription in ovine endometrial epithelial cells. Endocrinology 142:98–107 [DOI] [PubMed] [Google Scholar]
- 14.Rosenfeld CS, Han CS, Alexenko AP, Spencer TE, Roberts RM2002. Expression of interferon receptor subunits, IFNAR1 and IFNAR2, in the ovine uterus. Biol Reprod 67:847–853 [DOI] [PubMed] [Google Scholar]
- 15.Song G, Spencer TE, Bazer FW2006. Progesterone and interferon-τ regulate cystatin C in the endometrium. Endocrinology 147:3478–3483 [DOI] [PubMed] [Google Scholar]
- 16.Marcotte R, Zhou L, Kim H, Roskelly CD, Muller WJ2009. c-Src associates with ErbB2 through an interaction between catalytic domains and confers enhanced transforming potential. Mol Cell Biol 29:5858–5871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy LO, Blenis J2006. MAPK signal specificity: the right place at the right time. Trends Biochem Sci 31:268–275 [DOI] [PubMed] [Google Scholar]
- 18.Bauer I, Hohl M, Al-Sarraj A, Vinson C, Thiel G2005. Transcriptional activation of the Egr-1 gene mediated by tetradecanoylphorbol acetate and extracellular signal-regulated protein kinase. Arch Biochem Biophys 438:36–52 [DOI] [PubMed] [Google Scholar]
- 19.Song G, Spencer TE, Bazer FW2005. Cathepsins in the ovine uterus: regulation by pregnancy, progesterone, and interferon τ. Endocrinology 146:4825–4833 [DOI] [PubMed] [Google Scholar]
- 20.Arosh JA, Banu SK, Kimmins S, Chapdelaine P, Maclaren LA, Fortier MA2004. Effect of interferon-τ on prostaglandin biosynthesis, transport, and signaling at the time of maternal recognition of pregnancy in cattle: evidence of polycrine actions of prostaglandin E2. Endocrinology 145:5280–5293 [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Wu X, Gui P, Wu J, Sheng JZ, Ling S, Braun AP, Davis GE, Davis MJ2010. α5β1 Integrin engagement increases large conductance, Ca2+ activated K+ channel current and Ca2+ sensitivity through c-src-mediated channel phosphorylation. J Biol Chem 285:131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burghardt RC, Johnson GA, Jaeger LA, Ka H, Garlow JE, Spencer TE, Bazer FW2002. Integrins and extracellular matrix proteins at the maternal-fetal interface in domestic animals. Cells Tissues Organs 172:202–217 [DOI] [PubMed] [Google Scholar]
- 23.Zhou F, You G2007. Molecular insights into the structure-function relationship of organic anion transporters OATs. Pharm Res 24:28–36 [DOI] [PubMed] [Google Scholar]
- 24.Takano M, Nagai J, Yasuhara M, Inui K1996. Regulation of p-aminohippurate transport by protein kinase C in OK kidney epithelial cells. Am J Physiol Renal Physiol 271:F469–F475 [DOI] [PubMed]
- 25.Huff RA, Vaughan RA, Kuhar MJ, Uhl GR1997. Phorbol esters increase dopamine transporter phosphorylation and decrease transport Vmax. J Neurochem 68:225–232 [DOI] [PubMed] [Google Scholar]
- 26.Hesse D, Sauvant C, Holzinger H, Gekle M2004. Apical expression or expression in a non polarized cell of hOAT1 inverses regulation by epidermal growth factor (EGF) as compared to basolateral hOAT1. Cell Physiol Biochem 14:177–186 [DOI] [PubMed] [Google Scholar]
- 27.Sauvant C, Hesse D, Holzinger H, Evans KK, Dantzler WH, Gekle M2004. Action of EGF and PGE2 on basolateral organic anion uptake in rabbit proximal renal tubules and hOAT1 expressed in human kidney epithelial cells. Am J Physiol Renal Physiol 286:F774–F783 [DOI] [PubMed]
- 28.Soodvilai S, Wright SH, Dantzler WH, Chatsudthipong V2005. Involvement of tyrosine kinase and PI3K in the regulation of OAT3-mediated estrone sulfate transport in isolated rabbit renal proximal tubules. Am J Physiol Renal Physiol 289:F1057–F1064 [DOI] [PubMed]
- 29.You G2004. Towards an understanding of organic anion transporters: structure-function relationships. Med Res Rev 24:762–774 [DOI] [PubMed] [Google Scholar]
- 30.Fleming JG, Spencer TE, Safe SH, Bazer FW2006. Estrogen regulates transcription of the ovine oxytocin receptor gene through GC-rich SP1 promoter elements. Endocrinology 147:899–911 [DOI] [PubMed] [Google Scholar]
- 31.Fernández-Alvarez A, Tur G, López-Rodas G, Casado M2008. Reciprocal regulation of the human sterol regulatory element binding protein (SREBP)-1a promoter by Sp1 and EGR-1 transcription factors. FEBS Lett 582:177–184 [DOI] [PubMed] [Google Scholar]
- 32.Raychowdhury R, Schäfer G, Fleming J, Rosewicz S, Wiedenmann B, Wang TC, Höcker M2002. Interaction of early growth response protein 1 (Egr-1), specificity protein 1 (Sp1), and cyclic adenosine 3`5`-monophosphate response element binding protein (CREB) at a proximal response element is critical for gastrin-dependent activation of the chromogranin A promoter. Mol Endocrinol 16:2802–2818 [DOI] [PubMed] [Google Scholar]
- 33.Al-Sarraj A, Day RM, Thiel G2005. Specificity of transcriptional regulation by the zinc finger transcription factors Sp1, Sp3, and Egr-1. J Cell Biochem 94:153–167 [DOI] [PubMed] [Google Scholar]
- 34.Johnson GA, Burghardt RC, Newton GR, Bazer FW, Spencer TE1999. Development and characterization of immortalized ovine endometrial cell lines. Biol Reprod 61:1324–1330 [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Antoniou E, Liu Z, Hearne LB, Roberts RM2007. A microarray analysis for genes regulated by interferon-τ in ovine luminal epithelial cells. Reproduction 134:123–135 [DOI] [PubMed] [Google Scholar]
- 36.Banu SK, Lee J, Speights Jr VO, Starzinski-Powitz A, Arosh JA2009. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 induces apoptosis of human endometriotic cells through suppression of ERK1/2, AKT, NFkB and b-catenin pathways and activation of intrinsic apoptotic mechanisms. Mol Endocrinol 23:1291–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arosh JA, Banu SK, Chapdelaine P, Emond V, Kim JJ, MacLaren LA, Fortier MA2003. Molecular cloning and characterization of bovine prostaglandin E2 receptors EP2 and EP4: expression and regulation in endometrium and myometrium during the estrous cycle and early pregnancy. Endocrinology 144:3076–3091 [DOI] [PubMed] [Google Scholar]