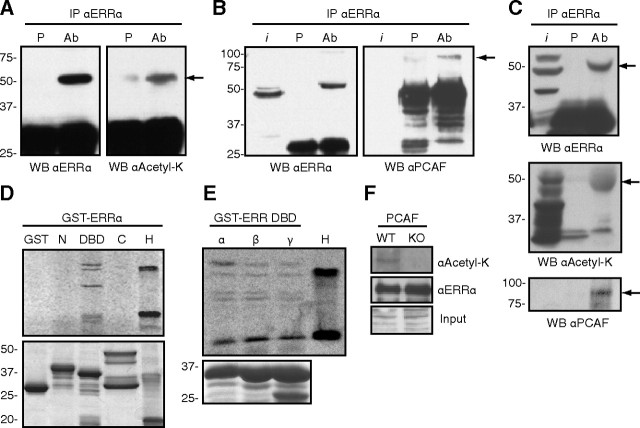
Fig. 1.
ERRα is an acetylated protein that interacts with and is acetylated by PCAF on the DBD. Panel A, ERRα was transiently transfected into 293 cells, immunoprecipitated with either preimmune serum (P) or anti-ERRα serum [antibody (Ab)], and blotted with an antibody directed against ERRα or a pan-antiacetylated lysine antibody. Arrow points to ERRα. Panel B, In a similar experiment the immunoprecipitated proteins were blotted with antibodies for ERRα or PCAF. Arrow points to PCAF; i, input. Panel C, ERRα interacts with PCAF and is acetylated in vivo. Lysate from mouse liver was immunoprecipitated with either preimmune serum (P) or anti-ERRα serum (Ab) and blotted for ERRα (top panel), acetylated lysine (middle panel), or PCAF (lower panel). Arrows point to ERRα (top and middle panels) and PCAF (lower panel). Panel D, In vitro acetylation assays were performed on bacterially expressed GST-tagged proteins encoding different domains of ERRα using a recombinant PCAF and 14C-radiolabeled acetyl-CoA substrate. N, Amino-terminal domain (amino acids 1–74; 34.7 kDa), DBD (amino acids 68–173; 37.6 kDa but migrates slightly faster due to the covalent zinc fingers structure), C, Carboxy-terminal domain (amino acids 165–423; 54.5 kDa). GST and histones (H) were used, respectively, as negative and positive controls. Upper panel is the acetylation assay, and lower is Coomassie staining to show equal protein loading. Panel E, A similar in vitro acetylation assay was performed with GST-DBD fusion proteins of the three members of the ERR family (ERRα/β/γ). H, histones. Bottom panel is Coomassie staining to show equal protein loading. Panel F, GST-tagged full-length ERRα was transiently expressed in immortalized wild-type (WT) or PCAF-null [knockout (KO)] MEFs. Equal amounts of saturated GST agarose beads were immunoblotted with an ERRα or pan-antiacetyl-lysine antibody. WB, Western blot.