Abstract
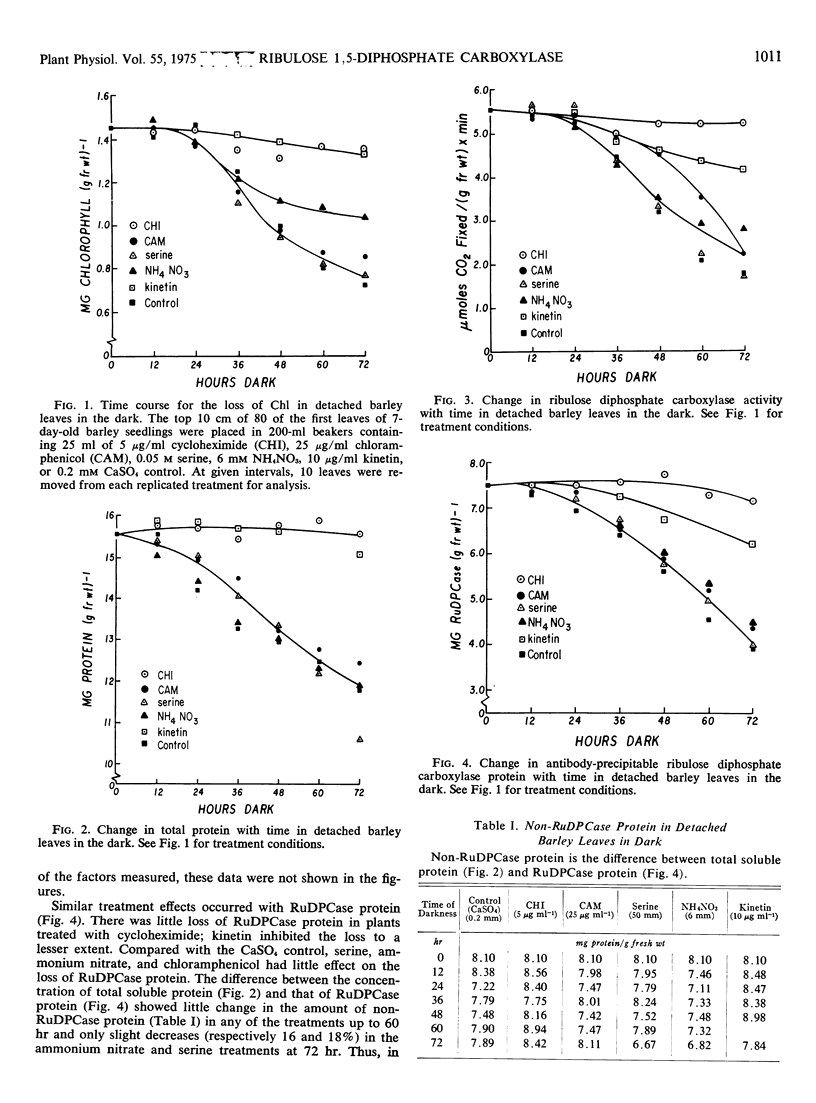
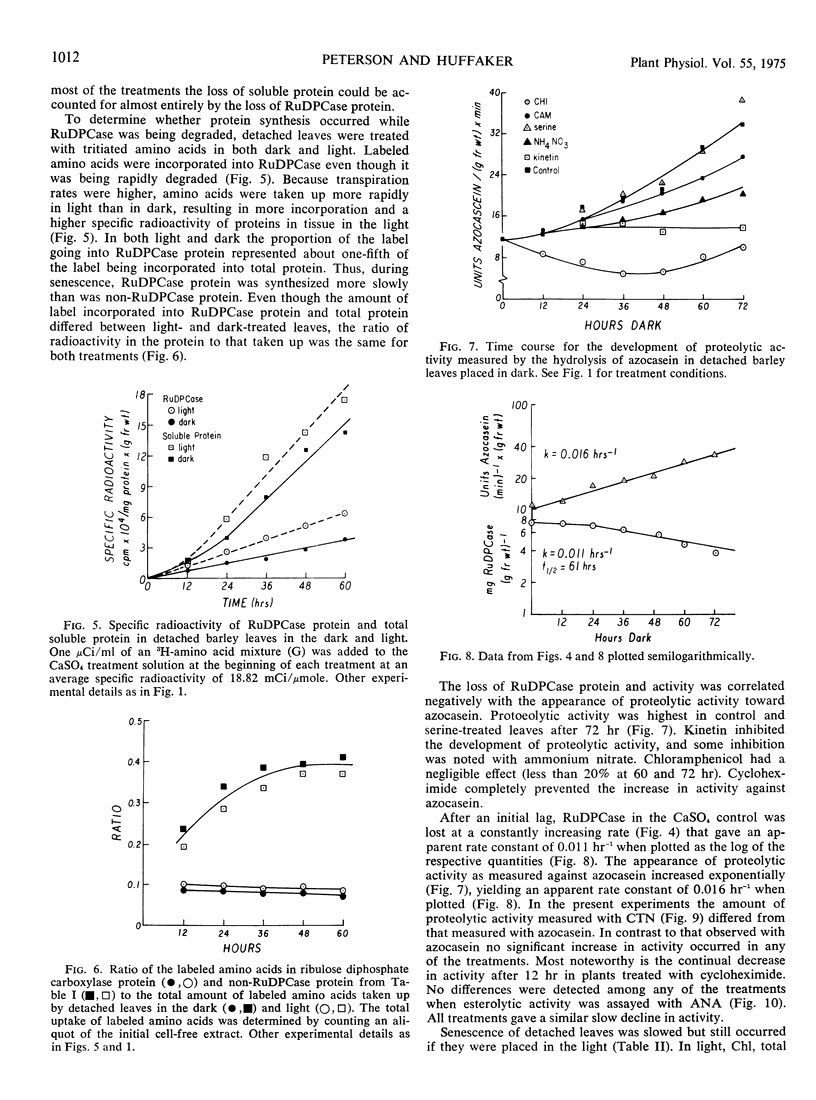
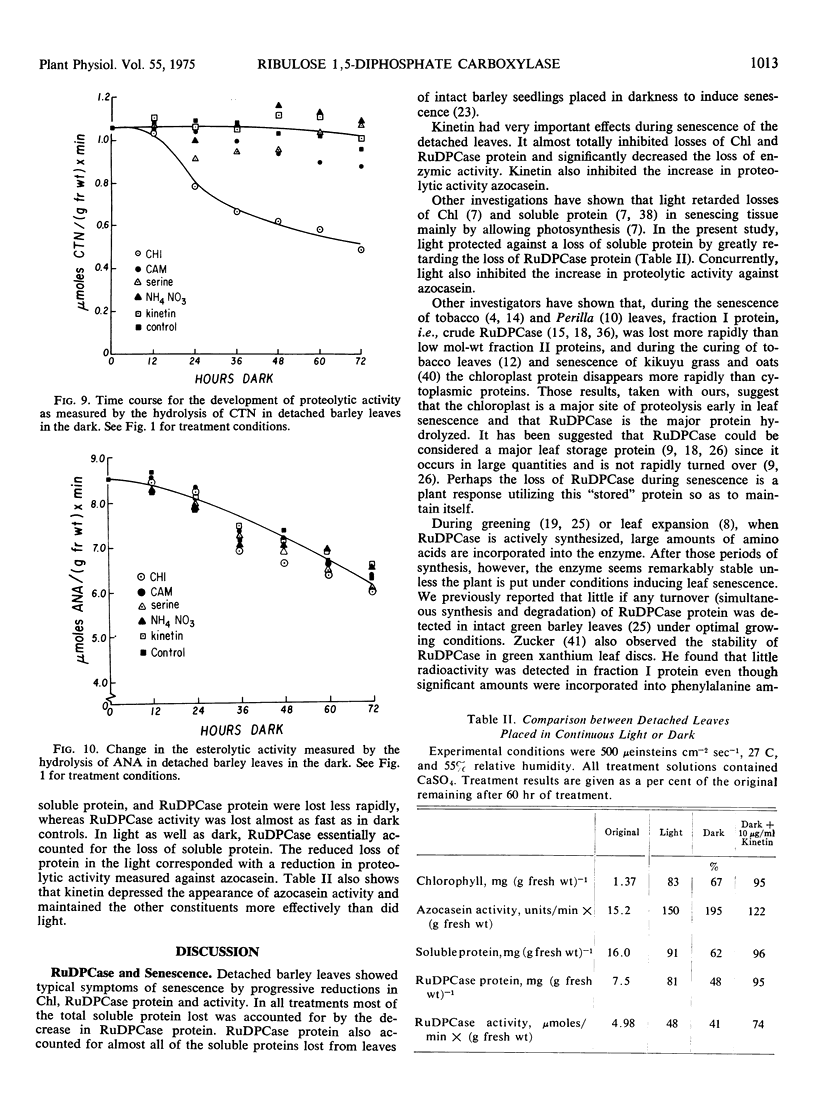
Symptoms typical of senescence occurred in green detached primary barley (Hordeum vulgare L.) leaves placed in darkness and in light. Chlorophyll, total soluble protein, ribulose 1,5-diphosphate carboxylase protein and activity each progressively decreased in darkness and to a lesser extent in light. In all treatments most of the total soluble protein lost was accounted for by a decrease in ribulose 1,5-diphosphate carboxylase protein, suggesting that the chloroplast was a major site of degradation early in senescence.
Loss of ribulose 1,5-diphosphate carboxylase protein was negatively correlated with an increase in proteolytic activity measured against azocasein. Both rates were exponential, with about a 30% difference in apparent rate constants. Cycloheximide essentially prevented the loss of chlorophyll, ribulose 1,5-diphosphate carboxylase protein, and activity and completely inhibited the increase in proteolytic activity against azocasein. Since chloramphenicol had little effect on the loss of ribulose 1,5-diphosphate carboxylase protein or chlorophyll, or on proteolytic activity against azocasein, it is suggested that the proteolytic activity was developed on cytoplasmic 80 S ribosomes.
Kinetin greatly retarded the onset of such symptoms of senescence by inhibiting the losses of chlorophyll and ribulose 1,5-diphosphate carboxylase protein and protected against inactivation of enzymic activity. It also prevented the increase in proteolytic activity measured against azocasein. Incorporation of labeled amino acids into ribulose 1,5-diphosphate carboxylase during its rapid degradation showed that the enzyme was under turnover. The changes in ribulose 1,5-diphosphate carboxylase protein and activity, chlorophyll, soluble protein other than ribulose 1,5-diphosphate carboxylase, proteolytic and esterolytic activity during senescence indicate that senescence is a selective, sequential process.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Criddle R. S., Dau B., Kleinkopf G. E., Huffaker R. C. Differential synthesis of ribulosediphosphate carboxylase subunits. Biochem Biophys Res Commun. 1970 Nov 9;41(3):621–627. doi: 10.1016/0006-291x(70)90058-6. [DOI] [PubMed] [Google Scholar]
- DORNER R. W., KAHN A., WILDMAN S. G. The proteins of green leaves. VII. Synthesis and decay of the cytoplasmic proteins during the life of the tobacco leaf. J Biol Chem. 1957 Dec;229(2):945–952. [PubMed] [Google Scholar]
- Epstein E. The essential role of calcium in selective cation transport by plant cells. Plant Physiol. 1961 Jul;36(4):437–444. doi: 10.1104/pp.36.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldthwaite J. J., Laetsch W. M. Regulation of senescence in bean leaf discs by light and chemical growth regulators. Plant Physiol. 1967 Dec;42(12):1757–1762. doi: 10.1104/pp.42.12.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker R. C., Obendorf R. L., Keller C. J., Kleinkopf G. E. Effects of Light Intensity on Photosynthetic Carboxylative Phase Enzymes and Chlorophyll Synthesis in Greening Leaves of Hordeum vulgare L. Plant Physiol. 1966 Jun;41(6):913–918. doi: 10.1104/pp.41.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp J. D., Sutton D. W. Protein metabolism in cultured plant tissues. Calculation of an absolute rate of protein synthesis, accumulation, and degradation in tobacco callus in vivo. Biochemistry. 1971 Jan 5;10(1):81–88. doi: 10.1021/bi00777a013. [DOI] [PubMed] [Google Scholar]
- Kleinkopf G. E., Huffaker R. C., Matheson A. A simplified purification and some properties of ribulose 1,5-diphosphate carboxylase from barley. Plant Physiol. 1970 Aug;46(2):204–207. doi: 10.1104/pp.46.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinkopf G. E., Huffaker R. C., Matheson A. Light-induced de Novo Synthesis of Ribulose 1,5-Diphosphate Carboxylase in Greening Leaves of Barley. Plant Physiol. 1970 Sep;46(3):416–418. doi: 10.1104/pp.46.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martin C., Thimann K. V. The role of protein synthesis in the senescence of leaves: I. The formation of protease. Plant Physiol. 1972 Jan;49(1):64–71. doi: 10.1104/pp.49.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. W., Kleinkopf G. E., Huffaker R. C. Evidence for lack of turnover of ribulose 1,5-diphosphate carboxylase in barley leaves. Plant Physiol. 1973 Jun;51(6):1042–1045. doi: 10.1104/pp.51.6.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rains D. W. Kinetics and Energetics of Light-enhanced Potassium Absorption by Corn Leaf Tissue. Plant Physiol. 1968 Mar;43(3):394–400. doi: 10.1104/pp.43.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J. A. Hormonal Control of Senescence of Bean Endocarp: Auxin-suppression of RNase. Plant Physiol. 1969 Feb;44(2):313–314. doi: 10.1104/pp.44.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J. A. Senescence: action of auxin and kinetin in control of RNA and protein synthesis in subcellular fractions of bean endocarp. Plant Physiol. 1967 Oct;42(10):1334–1342. doi: 10.1104/pp.42.10.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibaoka H., Thimann K. V. Antagonisms between Kinetin and Amino Acids: Experiments on the Mode of Action of Cytokinins. Plant Physiol. 1970 Aug;46(2):212–220. doi: 10.1104/pp.46.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis R. L., Jordan W. R., Huffaker R. C. Evidence for an Inactivating System of Nitrate Reductase in Hordeum vulgare L. during Darkness That Requires Protein Synthesis. Plant Physiol. 1969 Aug;44(8):1150–1156. doi: 10.1104/pp.44.8.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A. Control of the Protein Turnover Rates in Lemna minor. Plant Physiol. 1972 Jan;49(1):47–51. doi: 10.1104/pp.49.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON NOORT, WILDMAN S. G. PROTEINS OF GREEN LEAVES. IX. ENZYMATIC PROPERTIES OF FRACTION-I PROTEIN ISOLATED BY A SPECIFIC ANTIBODY. Biochim Biophys Acta. 1964 Aug 19;90:309–317. [PubMed] [Google Scholar]
- Wollgiehn R. Nucleic acid and protein metabolism of excised leaves. Symp Soc Exp Biol. 1967;21:231–246. [PubMed] [Google Scholar]


