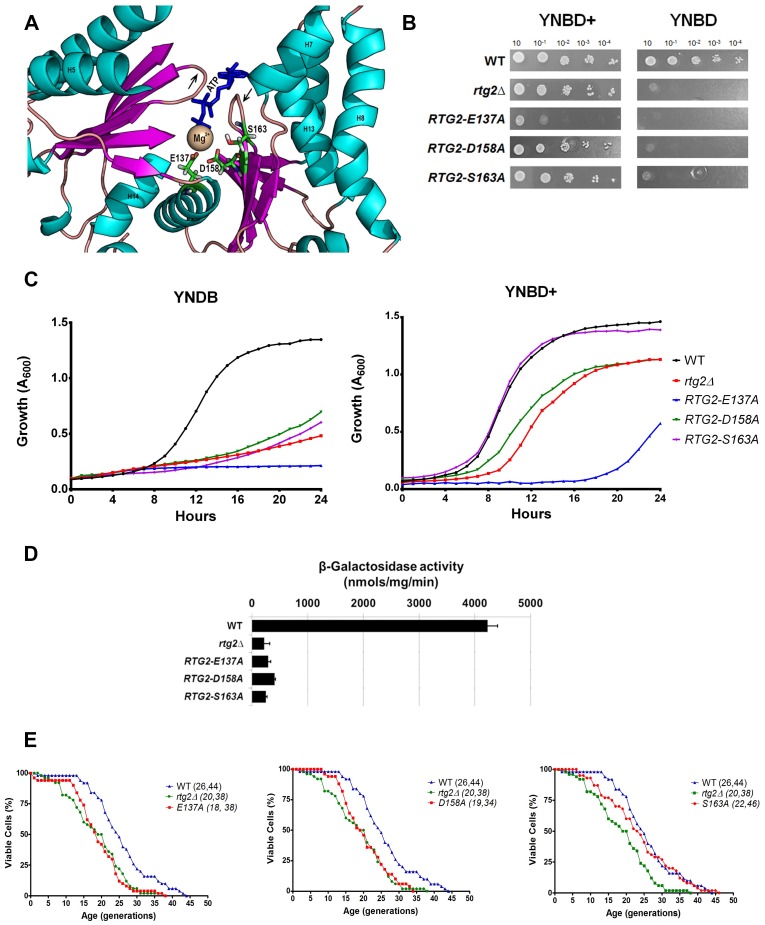
Fig 6. E137, D158, and S163 residues are likely involved in the coordination of Mg2+ ion at the ATP binding site of Rtg2p.
(A) Location of E137, D158, and S163 residues on ATP binding site of Rtg2p protein structural model. ATP-binding loops are indicated with arrows. (B) Mutations E137A, D158A, and S163A on Rtg2p cause glutamate auxotrophy to yeast cells. Glutamate auxotrophy assays were performed by spotting serial dilutions of cells on solid minimal media YNBD and YNBD+ (YNBD plus glutamate at 0.02%). (C) Growth performance of mutant strains on YNBD liquid medium are similar to that of rtg2Δ strain. Wild type, rtg2Δ and RTG2-E137A, RTG2-D158A, and RTG2-S163A strains were grown on YPD until saturation, diluted to A600 = 0.1 in either YNBD or YBND+, and incubated at 30°C, 160 rpm, for 24 h to monitor growth in a INFINITY PRO 200 (Tecan) microplate reader. (D) Retrograde signaling is not activated in RTG2-E137A, RTG2-D158A, and RTG2-S163A mutant strains. Wild type, rtg2Δ and point mutants were grown on YNBD medium until A600 = 0.6 and cell extracts were prepared to analyze CIT2-LacZ expression. β-galactosidase assays were performed as previously described (see Materials and Methods), and the activity normalized by total protein concentration. (E) Rtg2p mutations E137A (left panel) and D158A (middle panel) but not S163A (right panel) decrease the replicative lifespan of yeast. Fifty cells of each strain were aligned on YPD and daughter cells were removed from mothers to construct survival curves from at least two independent experiments. The mean and maximum lifespan are indicated between parentheses (mean, maximum). Statistical significance are summarized in Table 2.