Abstract
Craniocervical artery dissection is a potentially disabling condition caused by an intimal tear allowing blood to enter and dissect the media in the cranial direction which can occur spontaneously or as a result of trauma. When the dissection extends toward the adventitia, it can form a protrusion from the weakened vessel wall called a pseudoaneurysm, which may become a nidus for distal thromboembolism or cause mass effect on adjacent structures. Accurate and prompt diagnosis is critical as timely treatment can significantly reduce the risk of complications such as stroke. Here, we present a case of cervical ICA dissection and pseudoaneurysm formation causing mass effect with resultant compressive ipsilateral hypoglossal nerve palsy.
Keywords: Internal carotid artery dissection, Pseudoaneurysm
Case report
A 56-year-old man with a past medical history of hypertension presented to the neurology clinic with a few days history of dysphagia characterized by a sensation that the left side of his throat “could not bring things down.” There was no other neurologic deficit. The patient’s blood pressure was elevated at 170/90 mm Hg. On physical examination, the tongue was slightly deviated to the left.
Subsequent MRI of the brain before and after the administration of IV gadolinium demonstrated a crescentic area of hyperintensity on unenhanced T1-weighted imaging overlying the cervical portion of the internal carotid artery, representing the false lumen of a dissection of the internal carotid artery (Fig. 1). This signal abnormality extended from 4 cm above the origin of the internal carotid artery to the level of the skull base. There was associated narrowing of the true vessel lumen. There was also an area of focal outpouching, consistent with a pseudoaneurysm (Fig. 2, Fig. 3 and 4), approximately 2 cm from the skull base abutting the expected course of the hypoglossal nerve.
Fig. 1.
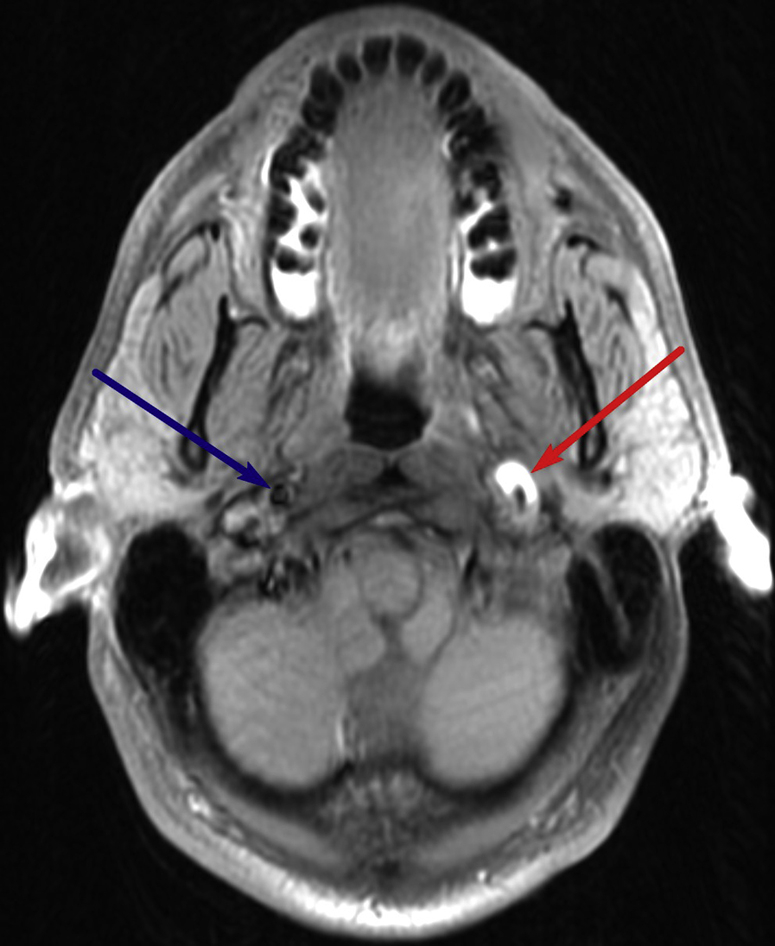
Pseudoaneurysm: Axial T1-weighted fat-saturated image through the upper neck demonstrates dissection of the left internal carotid artery cervical segment with narrowing of the flow-related signal void and a crescentic hyperintense rim (red arrow), compatible with hemorrhage in the subintimal space or false lumen. There is focal dilation at this level, likely representing a thrombosed pseudoaneurysm. The normal right ICA is also visualized (blue arrow). ICA, internal carotid artery.
Fig. 2.
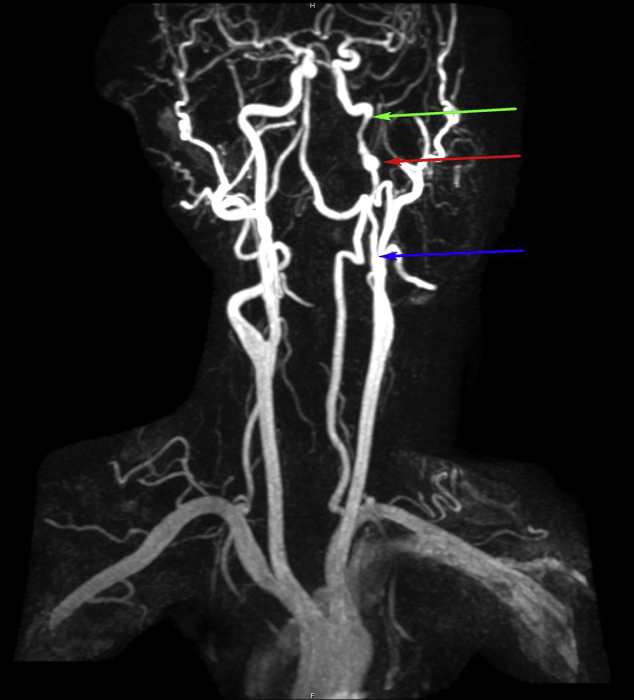
Coronal MIP reconstruction of a contrast-enhanced MRA of the carotid arteries demonstrates a segmental area of narrowing of the left cervical ICA, starting 4 cm from its origin (blue arrow), ending at the skull base (green arrow), with a focal area of aneurysmal dilation of the left internal carotid artery (red arrow) 2 cm from the skull base. MIP, maximum intensity projection; MRA, magnetic resonance angiogram.
Fig. 3.
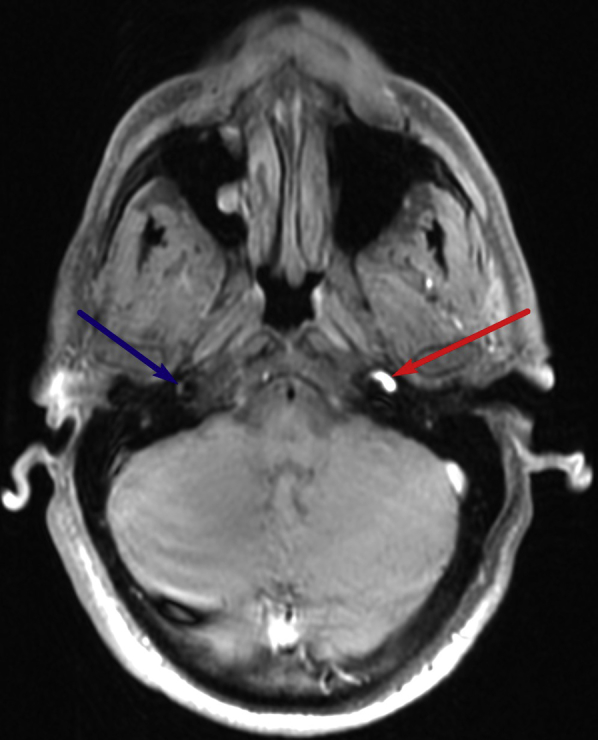
Proximal end of dissection: Axial T1-weighted fat-saturated image through the upper neck demonstrates dissection of the left cervical internal carotid artery, with narrowing of the flow-related signal void starting at 4 cm from the ICA origin. Again seen is a crescentic hyperintense rim (red arrow) surrounding the narrowed flow-related signal void of the cervical ICA, likely representing subacute hemorrhage within the false lumen. The normal right ICA is visualized (blue arrow).
Fig. 4.
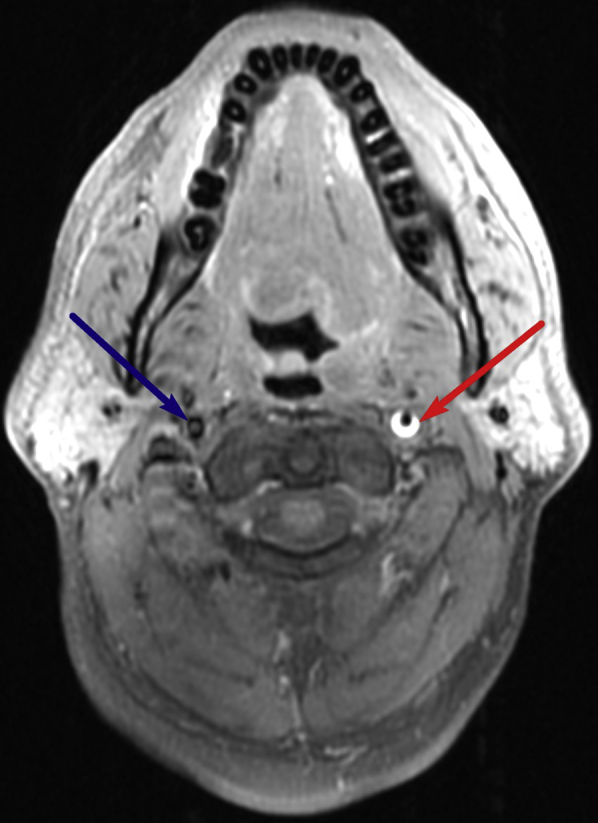
Distal end of dissection at skull base: Axial T1-weighted fat-saturated image through the upper neck demonstrates dissection of the left cervical internal carotid artery, with narrowing of the flow-related signal void starting at 4 cm from the ICA origin. Again seen is a crescentic hyperintense rim (red arrow) surrounding the narrowed flow-related signal void of the cervical ICA, likely representing subacute hemorrhage within the false lumen. The normal right ICA is visualized (blue arrow).
Based on the clinical and radiographic findings, the patient was diagnosed with a dissection of the cervical portion of the internal carotid artery, with pseudoaneurysm formation and compressive neuropathy of the hypoglossal nerve.
Discussion
Craniocervical artery dissection is a potentially disabling condition, affecting young and middle aged adults [1]. This is a relatively rare condition that is becoming increasingly recognized due to increasing use of neuroimaging techniques [1]. Craniocervical artery dissection may be traumatic or spontaneous and may be associated with underlying conditions like fibromuscular dysplasia or connective tissue diseases like Ehlers-Danlos or Marfan syndrome [2]. The presenting symptoms are often nonspecific and include headache, neck pain, or scalp tenderness. The sequelae can be catastrophic and include stroke, hemorrhage, or death [1].
Blood flow to the brain is mainly supplied by the craniocervical arteries which have walls composed of 3 layers including the inner intima, the muscular media of variable thickness, and the outer fibrous adventitia [1]. Injury to the arterial wall can result in craniocervical arterial dissection. This is a potentially disabling condition which may start as a primary intramural hematoma or as an intimal tear. This most commonly occurs at the extracranial segments of the carotid and vertebral arteries [1]. The dissection usually extends in the direction of blood flow thickening the injured wall. There is resulting narrowing of the lumen and enlargement of the external arterial diameter. When the dissection deepens, it may result in a pseudoaneurysm; a weakened focal outpouching contained by the thin adventitia (Table 1). The pseudoaneurysm formation time frame varies from hours to several years after intimal arterial injury with most presenting within 5 years [1]. The pseudoaneurysm can become a nidus for distal thromboembolism and predispose to stroke and can also cause mass effect on adjacent structures [1].
Table 1.
Summary Table—ICA pseudoaneurysm.
| Etiology | The normal wall of an arterial vessel includes three layers: the inner intima, the outer fibrous adventitia, and the muscular media in between. When the inner wall is compromised, a hematoma may form along the plane of the vessel wall. Patients with spontaneous dissection of the carotid artery are believed to have an underlying structural defect of the arterial wall. |
| Incidence | 2.6–2.9 per 100,000 |
| Gender ratio | No gender predilection although women usually present on average 5 years younger than males |
| Age predilection | 70% of patients with internal carotid arterial dissection are between the ages of 35 and 50 years, with a mean age of 47 years. |
| Risk factors | Conditions that have been involved in the pathogenesis are fibromuscular dysplasia, arteriopathies like cystic medial necrosis, hypertension, among others. |
| Treatment | Systemic anticoagulation (treatment of choice). Endovascular stenting/balloon dilation (persistent symptoms). |
| Prognosis | With spontaneous dissection, mortality is less than 5% with close to 75% of patients making a good recovery. With traumatic dissections, an estimated 37%–58% of patients have lasting neurological problems with higher mortality rates in comparison to patients with spontaneous dissection. |
Arterial dissections resulting in stenosis or near occlusion usually resolve with only mild residual mural disease. This is in contradistinction to dissections with total occlusion which may recanalize but often require intervention to return to their predissection caliber [1]. Treatment of a dissection usually begins with anticoagulation to prevent thrombosis and embolism [2]. However, anticoagulation increases the risk of subarachnoid hemorrhage if the dissection extends intracranially and is contraindicated in patients who present with subarachnoid hemorrhage [1]. More aggressive treatment, including endovascular or surgical intervention, may be used in patients who continue to remain symptomatic due to thromboembolic events and in patients who develop pseudoaneurysms [1].
Since craniocervical arterial dissection can have such catastrophic sequelae and complications, prompt diagnosis and management is crucial [3]. Digital subtraction angiography is considered the gold standard for diagnosis; however, this is an invasive technique and only assesses the lumen without determining the arterial wall thickness or configuration [1]. Ultrasound is an inexpensive and portable way of making the diagnosis with a faster acquisition time. Intramural hematoma formation would appear as a hyperechoic area within the vessel wall. Furthermore, if there is narrowing of the vessel lumen, duplex imaging will be able to identify that. However, since changes in vessel caliber are estimated in ultrasound based on flow velocity, the findings are inherently nonspecific because other conditions besides dissection can lead to increased or decreased flow velocities [1]. In the case presented, diagnosis was made using MRI and MRA imaging of the neck. In the acute and subacute setting, T1 fat-suppressed imaging of the neck is the sequence we use for diagnosis with intramural blood, usually in a crescent shape, demonstrating high signal intensity with adjacent narrowing of the flow-related signal void. Studies have shown that the sensitivity for dissection with MRI of the neck is 95% and specificity is 99% [1].
Other entities can mimic the radiographic findings of ICA dissection. These include entities such as fibromuscular dysplasia, dysgenesis of the ICA, atherosclerosis, neck irradiation treatment, Takayasu’s arteritis, Behcet’s disease, and Giant Cell arteritis [1]. The imaging features of these entities are summarized in Table 2.
Table 2.
Differential diagnosis.
| Imaging modality |
||||
|---|---|---|---|---|
| US/Doppler | MRI/MRA | CTA | Angiography | |
| Entity | ||||
| Fibromuscular dysplasia | Focal or long, tubular, multifocal stenosis with adjacent dilations. Diminished flow in narrowed areas. | Focal or long, tubular, multifocal stenosis with adjacent dilations. | Focal or long, tubular, multifocal stenosis with adjacent dilations. | Focal or long, tubular, multifocal stenosis with adjacent dilations. |
| Dysgenesis of ICA | Absence/hypoplasia of ICA. | Absence/hypoplasia of ICA. | Absence/hypoplasia of ICA. Absence of the carotid canal at the skull base. |
Absence/hypoplasia of ICA. |
| Atherosclerosis | Segmental narrowing of vessel lumen. Increased flow velocity through stenosis. | Segmental narrowing of vessel lumen. | Segmental narrowing of vessel lumen. | Segmental narrowing of vessel lumen. |
| Neck irradiation | Focal narrowing of vessel lumen. (Correlate with site of treatment). | Focal narrowing of vessel lumen. (Correlate with site of treatment). | Focal narrowing of vessel lumen. (Correlate with site of treatment). | Focal narrowing of vessel lumen. (Correlate with site of treatment). |
| Takayasu Arteritis | Bilateral areas of long segments of stenosis, occlusion, aneurysm formation. | Bilateral areas of long segments of stenosis, occlusion, aneurysm formation. | Bilateral areas of long segments of stenosis, occlusion, aneurysm formation. | Bilateral areas of long segments of stenosis, occlusion, aneurysm formation. |
| Behcet disease | Diminished flow, aneurysmal dilation. | Filling defects suggestive of occlusion, aneurysmal dilation. Often accompanied by abnormal signal (eg, edema, infarct, enhancement) in the midbrain. |
Filling defects suggestive of occlusion, aneurysmal dilation. | Filling defects suggestive of occlusion, aneurysmal dilation. |
| Giant cell arteritis | Diminished flow, areas of luminal narrowing or aneurysm formation. | Mural inflammation best demonstrated on T1 postcontrast. | Luminal abnormalities such as stenosis, occlusions, or aneurysm formation. | Luminal abnormalities such as stenosis, occlusions, or aneurysm formation. |
CTA, computed tomography angiography; US, ultrasound
Internal carotid artery pseudoaneurysms can cause complications, such as distal thromboembolism and can cause mass effect on adjacent structures. Consequences of dissection and pseudoaneurysm formation are dependent on the location of the vessel involved. Due to the location of the internal carotid artery, vessel enlargement can lead to lower cranial nerve palsies. Patients with pseudoaneurysms may have variable involvement of cranial nerve XII in conjunction with cranial nerves IX, X, and XI [4], [5]. Occasionally, there is an isolated palsy of cranial nerve XII as seen in our case [3].
The tongue palsy that results from damage to cranial nerve XII is the consequence of lower motor neuron damage [3]. The general consensus is that the palsy arises from compression or stretching of the nerve from the mass effect caused by the subadventitial hematoma in the wall of the internal carotid in the parapharyngeal space [6], [7], [8]. Chronic pulsation against the cranial nerve by the adjacent pseudoaneurysm is also a proposed mechanism for the palsy either independently or in conjunction with the mass effect. These effects can be successfully treated by endovascular coiling or surgical repair of the pseudoaneurysm. It has also been proposed that the underlying mechanism for the nerve palsy is ischemia from compression of the vessels supplying the nerve by the dissecting aneurysm [8]. If this is the case, then correcting a chronic pseudoaneurysm may have little or no effect.
The hypoglossal muscular and lingual branches supply all the ipsilateral intrinsic and extrinsic muscles of the tongue. Rarely in hypoglossal nerve damage, motor denervation may induce denervation pseudohypertrophy of the tongue, which is extensive fatty replacement, as opposed to a true hypertrophy where there is an increase in number or size of muscle fibers [9].
Ultimately, internal carotid artery dissection and pseudoaneurysm formation may be a debilitating condition; however, prompt imaging diagnosis can lead to early treatment and decreased complications, such as stroke and mass effect. Our patient was treated with steroids and low-molecular-weight heparin. He had symptomatic improvement, but a follow-up cervical angiogram after 8 weeks showed no change in the pseudoaneurysm which was then managed endovascularly by coil embolization.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Rodallec M., Marteau V., Gerber S., Desmottes L., Zins M. Craniocervical arterial dissection: spectrum of imaging findings and differential diagnosis. Radiographics. 2008;28:1723–1726. doi: 10.1148/rg.286085512. [DOI] [PubMed] [Google Scholar]
- 2.Kasravi N., Leung A., Silver I., Burneo J.G. Dissection of the internal carotid artery causing Horner syndrome and palsy of cranial nerve XII. CMAJ. 2010;182:E373–E377. doi: 10.1503/cmaj.091261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nusbaum A.O., Som P.M., Dubois P., Silvers A.R. Isolated vagal nerve palsy associated with a dissection of the extracranial internal carotid artery. AJNR. 1998;19:1845–1847. [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson E.O., Smoker W.R. Hypoglossal nerve palsy: a segmental approach. Radiographics. 1994;14:939–958. doi: 10.1148/radiographics.14.5.7991825. [DOI] [PubMed] [Google Scholar]
- 5.Badion M.L., Lim C.C., Teo J., Ong P.L., Hui F. Solitary fibrous tumor of the hypoglossal nerve. AJNR. 2003;24:343–345. [PMC free article] [PubMed] [Google Scholar]
- 6.Mokri B., Silbert P.L., Schievink W.I., Piepgras D.G. Cranial nerve palsy in spontaneous dissection of the extracranial internal carotid artery. Neurology. 1996;46:356–359. doi: 10.1212/wnl.46.2.356. [DOI] [PubMed] [Google Scholar]
- 7.Guy N., Deffond D., Gabrillargues J., Carriere N., Dordain G., Clavelou P. Spontaneous internal carotid artery dissection with lower cranial nerve palsy. Can J Neurol Sci. 2001;28:265–269. doi: 10.1017/s031716710000144x. [DOI] [PubMed] [Google Scholar]
- 8.Dennis M., Bowen W.T., Cho L. Elsevier; Australia: 2012. Mechanisms of Clinical Signs; pp. 396–400. [Google Scholar]
- 9.Holle D., Kastrup O., Sheu S.-Y., Obermann M. Tongue pseudohypertrophy in idiopathic hypoglossal nerve palsy. J Neurol Neurosurg Psychiatry. 2009;80:1393. doi: 10.1136/jnnp.2008.169938. [DOI] [PubMed] [Google Scholar]