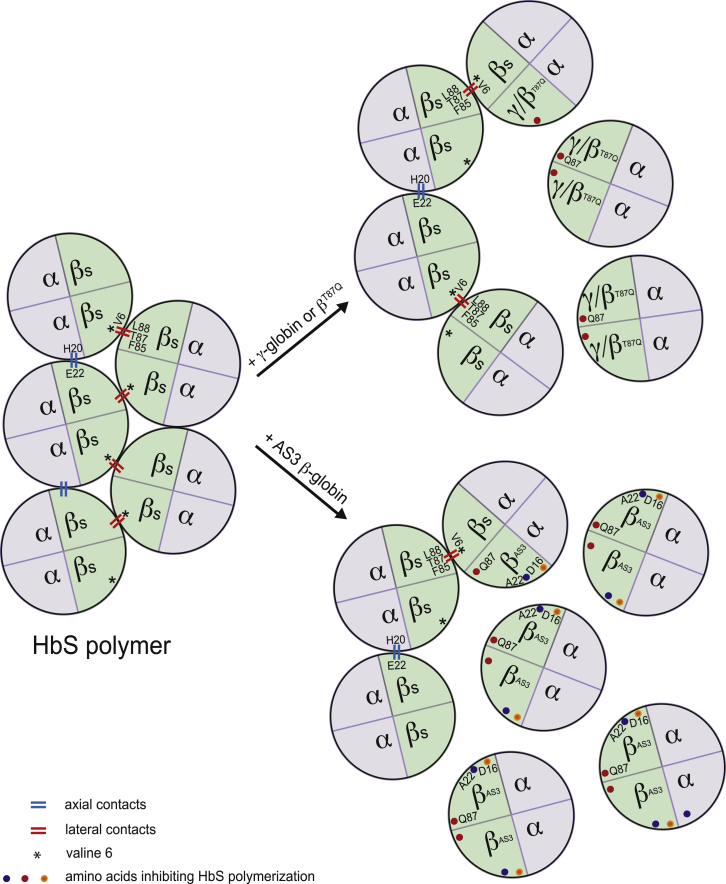
Figure 3.
Inhibition of HbS Polymerization by Anti-sickling β-like Globins
HbS tetramers polymerize upon de-oxygenation. In HbS polymers, the valine at position 6 (V6) of the βS-chain forms a lateral contact with the phenylalanine and leucine residues at positions 85 and 88 (F85 and L88) of the βS-chain of the adjacent tetramer. Additionally, a glutamic acid (at position 22) of the β-chain interacts with a histidine residue (at position 20) of the α-globin of a neighboring tetramer (axial contact). The incorporation of γ- or βT87Q-globin into the Hb prevents HbS polymerization. βT87Q-Globin harbors an amino acid substitution at position 87 (threonine [T87] to glutamine [Q87]). The glutamine residue derived from the γ-globin chain inhibits the formation of lateral contacts between the Hb tetramers. In addition to the T87Q amino acid substitution, in AS3 β-globin, the glutamic acid at position 22 is replaced by an alanine (A22), which disrupts the axial contacts between the Hb tetramers, and the glycine at position 16 is replaced by an aspartic acid (D16), thus increasing the affinity of the AS3 β-chain to the α-globin polypeptide.