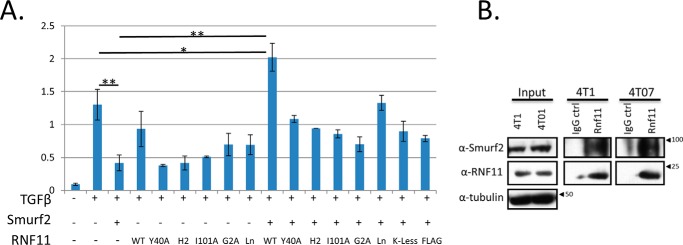
Figure 9.
RNF11 functionally antagonizes SMURF2 by sequestration in an endosomal compartment. A, SMURF2·RNF11 wild-type complex antagonizes SMURF2 repression of TGFβ-dependent transcriptional response. Mink lung epithelial cells stably expressing the TGFβ-responsive PAI-1 reporter fused to firefly luciferase were transfected with wild-type SMURF2, wild-type and mutant RNF11, and Renilla luciferase (to control for transfection efficiency) and serum-starved overnight, followed by treatment with recombinant TGFβ (400 pm for 16 h). The y axis represents relative light units as determined by the ratio of detected firefly luminescence to Renilla luminescence. Values represent means from at least two independent experiments, and error bars represent S.D. *, p < 0.02; **, p < 0.005. B, endogenous SMURF2·RNF11 complex is present in a mouse model for metastatic breast cancer. RNF11 immunoprecipitates using our in-house RNF11 antibody coupled to Protein A-Sepharose were prepared from 2 mg of crude cell lysates from 4T07 and 4T1 cells. IgG coupled to Protein A-Sepharose served as a control for nonspecific interactions in each case. The immunoprecipitates were resolved by SDS-PAGE, blotted, and then probed for endogenous RNF11 and SMURF2 (see “Experimental procedures”). Input blots were probed with anti-tubulin antibody.