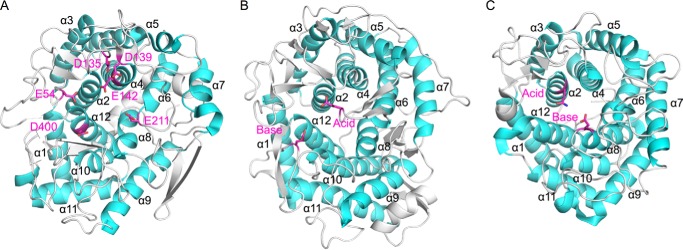
Figure 12.
Structural comparison with other GH family enzymes. The α-helices are colored cyan. Catalytic residues (GH15 and GH8) and strictly conserved amino acids in the active site of Cpin_6279 are colored magenta and shown as a stick model. A, overall structure of Cpin_6279rN. Glu-54, Asp-135, Asp-139, Glu-142, Glu-211, and Asp-400 are strictly conserved residues. Glu-54 is located between α1 and α2. Asp-135, Asp-139, and Glu-142 are located between α3 and α4. Glu-211 is located between α5 and α6. Asp-400 is located between α11 and α12. B, overall structure of GH15 glucoamylase (PDB code 1AGM). Glu-179 (catalytic acid) is located between α5 and α6. Glu-400 (catalytic base) is located between α11 and α12. C, overall structure of GH8 endoglucanase (PDB code 1KWF). Glu-95 (mutated to Gln-95, catalytic acid) is located between α1 and α2. Glu-278 (catalytic base) is located between α7 and α8.