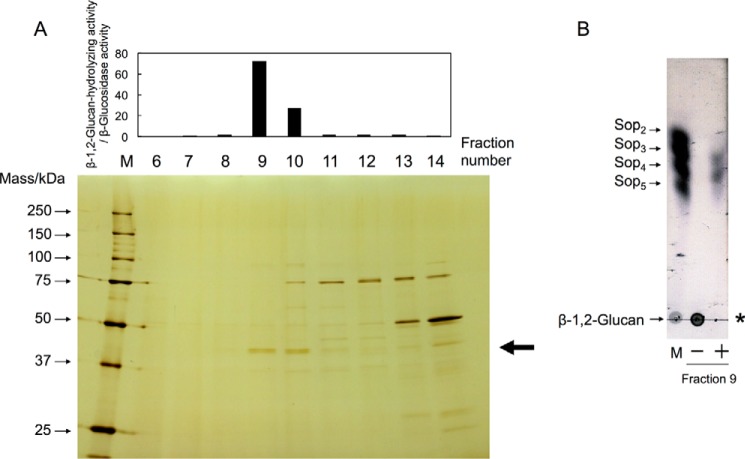
Figure 2.
Purification of endo-β-1,2-glucanase from a C. arvensicola cell extract. A, fractionated proteins were subjected to SDS-PAGE, followed by silver staining (bottom), and then the enzymatic activity was measured (top). Lane M contains protein standard markers. Endo-β-1,2-glucanase activity was evaluated as the ratio of β-1,2-glucan-hydrolyzing activity to β-glucosidase activity. The enzymatic reaction was conducted in a mixture (50 μl) containing 80% (v/v) enzyme fraction, 0.2% (w/v) β-1,2-glucan (average DP 64), and 50 mm MOPS-NaOH buffer (pH 6.5) at 30 °C for 16 h. An aliquot of the reaction mixture (40 μl) was mixed with 160 μl of a 1% (w/v) PAHBAH-HCl solution and then heated at 100 °C for 5 min, followed by measurement of absorbance at 405 nm. The arrow indicates the protein band subjected to N-terminal amino acid sequence analysis. B, TLC analysis of endo-β-1,2-glucanase in fraction 9. The enzymatic reaction was carried out in a mixture (10 μl) containing 85% (v/v) fraction 9 concentrated using Amicon Ultra 30,000 molecular weight cutoff (Millipore), 0.2% (w/v) β-1,2-glucan (average DP 64), and 50 mm MOPS-NaOH buffer (pH 6.5) at 30 °C for 16 h, and then an aliquot of the mixture was spotted onto a TLC plate. Lane M contains markers (1 μl of 0.2% (w/v) each sugar). The asterisk indicates the origin on the TLC plate. Minus and plus indicate whether fraction 9 was added to the reaction mixture or not.