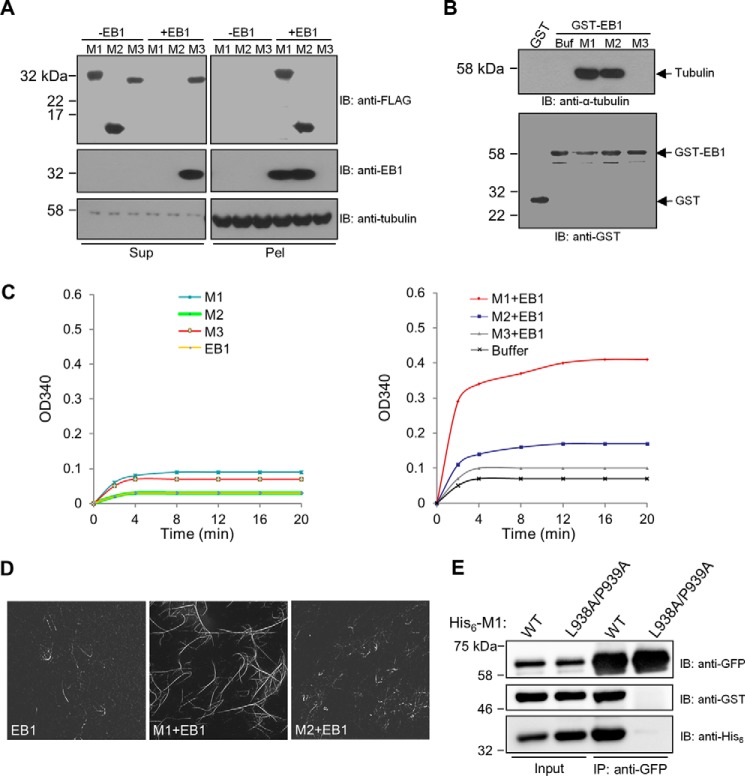
Figure 4.
Effects of SXIP proteins on EB1 activities toward microtubules and α/β-tubulin and on EB1 dimerization. A, a microtubule sedimentation assay was performed with the purified recombinant proteins of CDK5RAP2 fragments and EB1. In the assay, microtubules preassembled from 1 μm α/β-tubulin were incubated with the CDK5RAP2 fragments (His6-FLAG tagged; 0.5 μm) and EB1 (His6 tagged; 0.5 μm). After sedimentation, the resulting supernatants (Sup) and pellets (Pel) were analyzed by immunoblotting (IB) as indicated. B, the binding of GST and GST-EB1 to α/β-tubulin heterodimers was tested in a GST pulldown assay. α/β-Tubulin heterodimers (1 μm) were incubated with the GST proteins (1 μm) alone and in combination with different CDK5RAP2 fragments (His6-FLAG tagged; 1.25 μm). Buf, Buffer. The GST pulldown samples were analyzed by means of anti-α-tubulin and anti-GST immunoblotting. α/β-Tubulin heterodimers were coprecipitated with GST-EB1 in the presence of M1 or M2 but failed to coprecipitated with GST-EB1 alone or GST-EB1 plus M3. C, microtubule polymerization was conducted with α/β-tubulin heterodimers (18 μm) and microtubule seeds (0.1 μg/μl). The recombinant proteins of M1, M2, M3, and EB1 (0.5 μm) were applied alone or in combinations. D, microtubules were polymerized from a mixture of rhodamine-labeled and unlabeled α/β-tubulin as in C. The polymerized microtubules were examined by fluorescence microscopy. E, in a binding assay of purified recombinant proteins, His6-GFP-EB1 (0.09 μm) was incubated with GST-EB1 (0.16 μm) in the presence of wild-type His6-M1 (WT) or His6-M1(L938A/P939A) (2.5 μm). After anti-GFP immunoprecipitation (IP), the immunoprecipitates (50%) and the protein inputs (2%) were analyzed by means of immunoblotting. GST-EB1 was coprecipitated with His6-GFP-EB1 in the presence of wild-type M1 but not in the presence of the mutant.