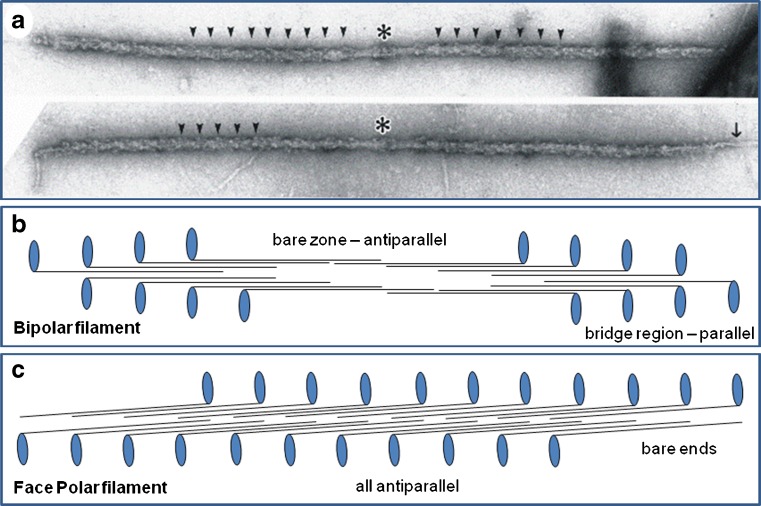
Fig. 2.
a Electron micrographs of isolated myosin filaments from bony fish muscle, adapted from AL-Khayat et al. (2008). The M-band is centrally located (asterisk) and some of the 43 nm crown repeats are indicated by arrowheads. The single arrow on the right indicates so-called end filaments which are part of the titin assembly at the tip of the myosin filaments (Trinick 1981). b Schematic diagram showing how myosin molecules pack together to give bipolar myosin filaments. Each myosin molecule is represented as a black line with a blue globular region on one end. Each blue oval represents the myosin head pair of one molecule. Rod packing is antiparallel in the middle of the filaments and parallel at each end. The parallel packing continues for very many crowns on each side of the central bare zone (the head-free region). Myosin molecules in one half of the filament have opposite polarity to those in the other half. This compares with the different packing scheme in (c) for the face polar myosin filaments in vertebrate smooth muscle. Here, the packing is antiparallel throughout and the molecules on opposite faces of the filaments point in opposite directions