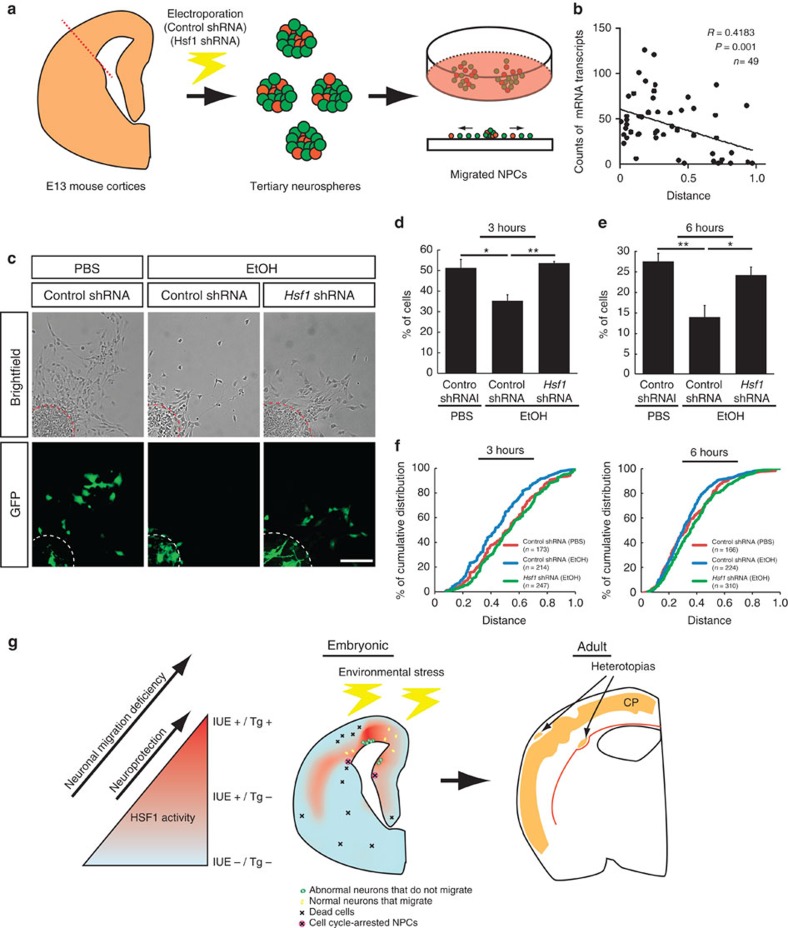
Figure 8. Reducing excessive Hsf1 activation mitigates EtOH-induced migration deficits.
(a) Schematic drawing of the migration assay. (b) The levels of Hsp70 expression (smFISH) are negatively correlated with the migration distance of cells from the center of the neurosphere after 3-hour culture. The position of the cell that had migrated the longest distance from the center (0.0) was set as 1.0, and was used for determining the relative positions of the other cells. (c) Representative images of the migration assays after a 6-h culture. Scale bar, 0.1 mm. (d,e) The percentages of GFP+ cells within the outer half of the maximum migration distance from the center of the neurosphere in total GFP+ cells. Four independent experiments were performed per group. Data are represented as mean±s.e.m. One-way ANOVA, F(2,9)=9.15, P=0.007 (d), F(2,9)=9.59 P=0.006 (e). *P<0.05, **P<0.01 by post hoc Tukey test. (f) Cumulative distribution of the cells shows the alleviation of EtOH-induced migration deficits by Hsf1 shRNA (n>150 cells from 4 biological replicates per group, P<0.05 by K-S test for all pairs except the pair of Control shRNA (PBS) (red) versus Hsf1 shRNA (EtOH)(green). (g) A model for the production of various cortical dysplasia due to probabilistic Hsf1–Hsp signalling activation elicited by prenatal environmental stressors. Excessive activation of HSF1 in a subpopulation of cortical cells, as detected in the reporter transgenic mouse (left), disrupts their normal developmental processes such as migration (green cells in middle panel). Moderate activation of the signalling that is detectable by IUE-based method, but not in the transgenic mouse (Fig. 3), serves to protect the cells from the environmental stressors15 (Fig. 2, left), and allows the cells develop normally (yellow cells in middle). Meanwhile, another subpopulation of cells may not have enough HSF1 activation to protect them from damage elicited by environmental challenges, thereby resulting in focal accumulation of dead cells or NPCs with impaired proliferation15 (Fig. 2, the cells indicated by X in middle panel). These heterogeneous events of abnormal development occur probabilistically (Fig. 1), accounting for individually distinct pattern of focal cortical malformations in the cortex exposed to similar levels of environmental challenges (right panel).