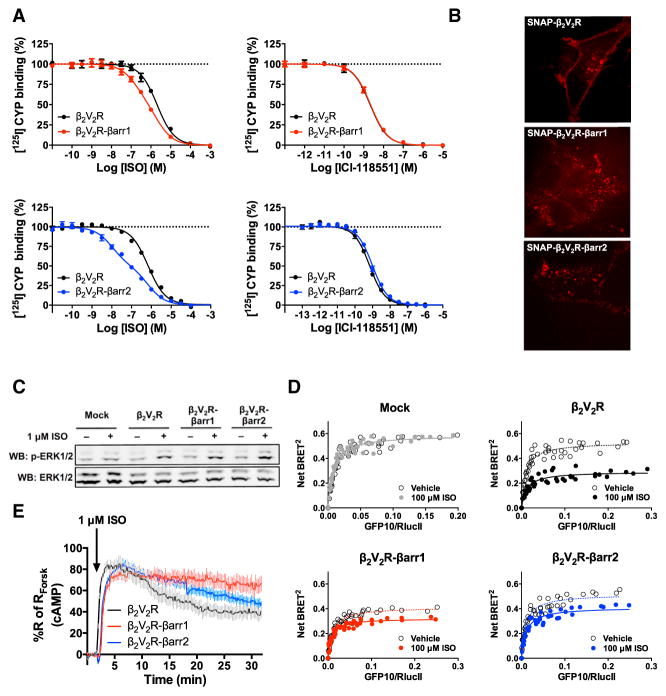
Figure 5. Functionality and Capability of β2V2R-βarr1/2 Fusions to Activate Gs in HEK293 Cells.
(A) Functional assessment of β2V2R-βarr1/2 fusions using radioligand competition binding experiments. Both agonist (ISO) and antagonist (ICI-118551) successfully competed off [125I]-CYP at β2V2R, β2V2R-βarr1 and β2V2R-βarr2. Data represent the mean ± SE of N = 3–4 experiments.
(B) Cellular localization of SNAP-β2V2R and SNAP-β2V2R-βarr1/2 fusions pre-labeled with SNAP-Surface 549 fluorescent substrate (549) using confocal microscopy (100× objective, N = 3 experiments, and n ≥ 16 cells).
(C) Characterization of 1 μM ISO-stimulated ERK1/2 phosphorylation response at 10 min post-stimulation in mock, β2V2R, β2V2R-βarr1, and β2V2R-βarr2-transfected cells (N = 6 experiments).
(D) ISO-stimulated Gs activation in mock (gray), β2V2R (black), β2V2R-βarr1 (red), and β2V2R-βarr2 (blue) transfected cells determined by BRET titration curves 30 min after stimulation (N = 4 experiments).
(E) Real-time cAMP measurement, utilizing HEK293-ICUE2 cells, in response to ISO-stimulation of β2V2R (black), β2V2R-βarr1 (red), and β2V2R-βarr2 (blue). Data represent the mean ± SE of N = 3 experiments and n ≥ 93 cells. Surface expression of GPCRs was matched in all experiments.
See also Figures S1 and S4.