Figure 6.
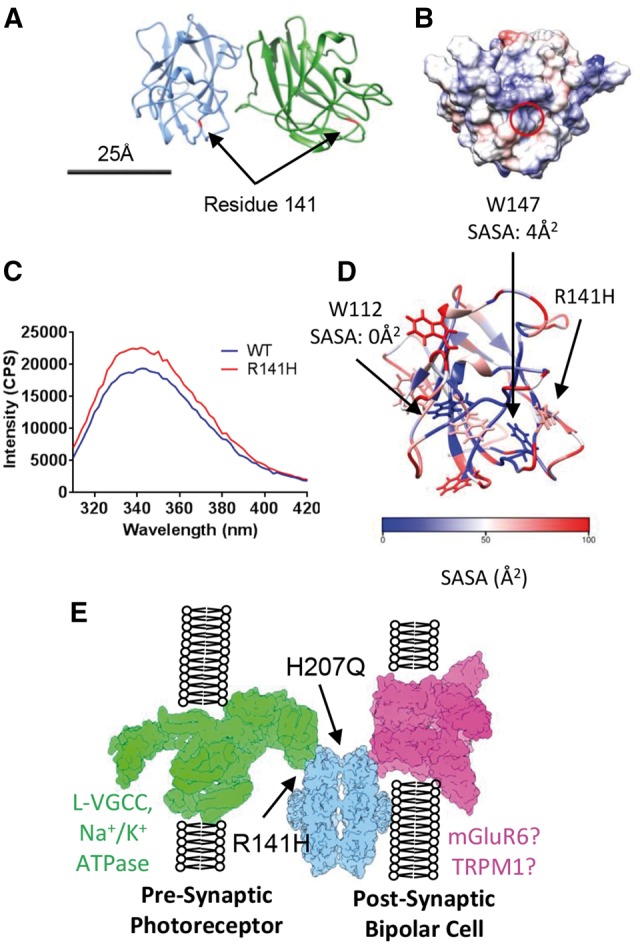
Mapping XLRS-causative conservative mutations onto the quasi-atomic model. (A) The position of residue R141 within the octamer is highlighted in red. (B) Electrostatic surface potential of the face of the discoidin domain containing residue R141 (circled) with overall positive charge. Scale bar for (A) and (B) = 25 nm. (C) Intrinsic fluorescence comparison of wild-type and R141H retinoschisin monomer. The R141H mutant has reduced fluorescence quenching suggesting increased solvent exposure of tryptophans. Shown is the emission spectrum after excitation at 295nm (n = 3). (D) Solvent accessible surface area (SASA) analysis of the tryptophans in the retinoschisin discoidin domain. W147 and W112 are buried and close to the R141H mutation site. (E) The paired octamer model for retinoschisin structural support between photoreceptor and bipolar cell synaptic membranes, with the sites affected by R141H and H207Q mutations labelled.
