Abstract
Lichen planus (LP) is an inflammatory skin condition with characteristic clinical and histopathological findings. Classic LP typically presents as pruritic, polygonal, violaceous flat-topped papules and plaques; many variants in morphology and location also exist, including oral, nail, linear, annular, atrophic, hypertrophic, inverse, eruptive, bullous, ulcerative, lichen planus pigmentosus, lichen planopilaris, vulvovaginal, actinic, lichen planus-lupus erythematosus overlap syndrome, and lichen planus pemphigoides. Clinical presentation of the rarer variant lesions may be largely dissimilar to classic LP and therefore difficult to diagnose based solely on clinical examination. However, histopathological examination of LP and LP-variant lesions reveal similar features, aiding in the proper diagnosis of the disease. Management of LP and LP variants aims to control symptoms and to decrease time from onset to resolution; it often involves topical corticosteroids, but varies depending on the severity and location of the lesion. The literature contains an array of reports on the variations in presentation and successful management of LP and its variants. A familiarity with LP and its variants is important in achieving timely recognition and management of the disease.
Keywords: lichen, planus, lichenoid, LP, planopilaris, variants
Introduction
Lichen planus (LP) is an inflammatory skin condition with characteristic clinical and histopathological findings that affects between 0.5 and 1% of the population. Classic LP typically presents as pruritic, polygonal, violaceous flat-topped papules and plaques; many variants in morphology and location also exist, including oral, nail, linear, annular, atrophic, hypertrophic, inverse, eruptive, bullous, ulcerative, LP pigmentosus, lichen planopilaris, vulvovaginal, actinic, lichen planus-lupus erythematosus overlap syndrome, and lichen planus pemphigoides. Many of the variants occur much more infrequently than classic LP. The rarity of the variants and their atypical presentations make their timely diagnosis and management more difficult in the clinical setting. Herein, we review classic LP and its many variants reported in the literature (Figs. 1–15).
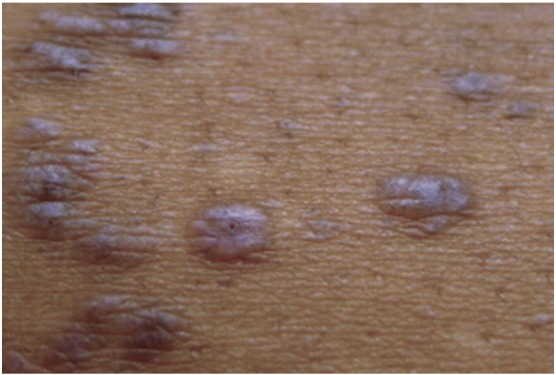
Classic LP lesions commonly present with the four P’s: purple, pruritis, polygonal, and papules/plaques. The papules often have dry, shiny surfaces with branny scale that forms fine, whitish streaks known as Wickham’s striae (Fig. 1, Fig. 2, Fig. 3). LP lesions are typically symmetric in distribution and can affect any area of the body, but LP tends to favor flexural surfaces of the forearms, wrists, and ankles; the dorsal surface of the hands; the shins; trunk; and sacral region. Involvement of the oral mucosa is also common. Lesions may involve other cutaneous (i.e., scalp, hair, and nails) and mucosal (i.e., genital, esophageal, and conjunctival) sites, but, interestingly, the face is rarely affected.
Fig. 1.
Lichen planus. Photo courtesy of Dr. Justin Finch, University of Connecticut Department of Dermatology.
Fig. 2.
Lichen planus. Photo courtesy of Dr. Justin Finch, University of Connecticut Department of Dermatology.
Fig. 3.
Lichen planus. Photo courtesy of Dr. Justin Finch, University of Connecticut Department of Dermatology.
LP affects patients of all ages, but up to 95% of all cases occur in adults, with most patients presenting between the third and sixth decades of life (Bilgili et al., 2012). While LP is generally considered an adult disease, 5 to 10% of cases do occur in children (Kumar et al., 1993, Luis-Montoya et al., 2005), the majority of which are reported in India (Handa and Sahoo, 2002, Kanwar and De, 2010, Kumar et al., 1993, Pandhi et al., 2014, Sharma and Maheshwari, 1999). In the childhood population, onset is more common in school-aged children, with mean reported age ranging from 7 to 11.8 years old, though infantile cases have rarely been reported (Balasubramaniam et al., 2008, Handa and Sahoo, 2002, Kanwar and De, 2010, Luis-Montoya et al., 2005, Nanda et al., 2001, Nnoruka, 2007, Pandhi et al., 2014, Walton et al., 2010).
While no specific gender predominance has been recognized, LP may affect more adult females than adult males (Kyriakis et al., 2006). Among childhood LP studies, no gender prevalence has been detected (Balasubramaniam et al., 2008, Handa and Sahoo, 2002, Luis-Montoya et al., 2005, Nanda et al., 2001, Pandhi et al., 2014, Walton et al., 2010). In general, LP has not been shown to have a racial predilection; however, certain populations seem to have a higher incidence of the disease. In a U.S. study (Walton et al., 2010), a statistically significant greater number of LP patients were African American (72%) compared to the general clinic population (21%). LP may also be more common is patients of Indian descent, as five of the nine large childhood LP studies were conducted in India, where relatively large numbers of patients were detected in short periods of time. Similarly, a study from the United Kingdom found that children originating from the Indian subcontinent represented 80.8% of children with LP, but only 28% of the city’s general child population (Balasubramaniam et al., 2008). There is also a predominance of LP diagnosis reported in children of Arab and Afro-Caribbean background.
The high proportion of cases reported among these populations suggests a genetic susceptibility for LP. This idea is further supported by cases of familial LP, reported in 1 to 4.3% of childhood LP series (Kanwar and De, 2010, Nanda et al., 2001). However, familial cases have not been reported in all series, and specific antigens have yet to be determined. Pathogenicity of LP lesions involves the autoimmune-mediated lysis of basal keratinocytes by CD8 + lymphocytes, though definitive etiological triggers are still unknown. Case reports in both adults and children have shown an association between LP and the following: chronic liver diseases such as chronic active hepatitis (particularly hepatitis C) and primary biliary cirrhosis; complication of hepatitis B vaccination; viral and bacterial antigens; trauma (via the Koebner phenomenon); metal ions; medications; and a variety of autoimmune diseases such as autoimmune thyroiditis, myasthenia gravis, alopecia areata, vitiligo, thymoma, and autoimmune polyendocrinopathy (Luis-Montoya et al., 2005, Pandhi et al., 2014).
Histopathological features are consistent for LP and its variants. Light microscopic evaluation of LP lesions shows circumscribed, wedge-shaped hypergranulosis in the epidermis; marked hyperkeratosis; and irregular sawtooth-like acanthosis of rete ridges. The dermal–epidermal junction typically shows signs of vacuolar degeneration with apoptotic keratinocytes, while the upper dermis characteristically contains a dense, band-like lymphocytic infiltrate that can obscure the dermal–epidermal junction. Civatte bodies, hypothesized to be apoptotic keratinocytes ready for phagocytosis, can be seen in the epithelium and upper dermis. Direct immunofluorescence often reveals a large number of IgM-staining cytoid bodies in the dermal papillae or peribasilar areas.
In general, management of classic cutaneous LP depends on the location and severity of lesions and is largely based on clinical experience. Most cutaneous LP lesions resolve spontaneously within a few years. Goals of treatment include shortening the time from onset to resolution and reducing symptoms, especially pruritus. Topical corticosteroids (TCS), usually class I or II TCS, are the first line of therapies and typically suffice to alleviate symptoms. In cases where TCS fail to control pruritus, narrow-band UVB (NBUVB) often works well. Other second-line therapies include topical and systemic retinoids; systemic steroids; and topical tacrolimus, metronidazole, griseofulvin, and cyclosporine. Third-line therapies, including psoralen plus UVA (PUVA), thalidomide, mycophenolate mofetil, low molecular weight heparin, and iontophoresis, have also been reported as effective (Rashid et al., 2008). Systemic treatments are usually reserved for severe recalcitrant cases and are typically used in combination with TCS or phototherapy. Post-inflammatory hyperpigmentation is often seen after treatment and may take years to fade.
The various cutaneous and mucosal variants of lichen planus will be discussed below.
Oral lichen planus
Oral lichen planus (OLP) is a common presentation of lichen planus that can occur alone, but often occurs concomitantly with skin lesions. The oral form affects females more than males and commonly affects patients of middle age (Wagner et al., 2013). Exact prevalence is unknown, but has been estimated to be between 0.5% and 2.6% of various populations. Specific etiology is controversial, but there is strong evidence, as in classic LP, for immune-cell-mediated damage to basal keratinocytes of the mucosa. Evidence suggests that various adhesion molecules and cytokines including tumor necrosis factor alpha and RANTES (regulated on activation normal T cell expressed and secreted) may play roles in a complicated interaction among T cells, mast cells, and eosinophils. Premalignant potential, as well as the association with hepatitis C virus of OLP, are still both very controversial (DeRossi and Ciarrocca, 2005, Hiremath et al., 2011, Wagner et al., 2013).
OLP typically presents in several forms, which may appear alone or in combination: reticular, erosive, atrophic, and plaque-like. Reticular OLP is often asymptomatic and frequently is an incidental finding on examination. Characteristic reticular lesions are bilateral and symmetric with thin, slightly raised white lines or papules that form a lace-like pattern, most commonly on the buccal mucosa (Fig. 4). Reticular OLP can be erythematous or nonerythematous and may appear in an annular configuration. Erosive OLP lesions present as large areas of irregularly shaped ulcerations with whitish pseudomembranes in combination with areas of severely erythematous mucosa. Some lesions may appear with atrophy or radiating white striae at the junction between involved and uninvolved mucosa. Erosive lesions are typically painful and subject to irritation by dental hygiene products or foods. Other forms of OLP are less common. Differential diagnosis for OLP lesions includes autoimmune vesicobullous disorders, chronic ulcerative stomatitis, chronic graft versus host disease, lupus erythematosus, mucosal candidiasis, and oral hairy leukoplakia. As OLP lesions may clinically resemble other mucosal diseases, including malignancies, histopathological evaluation is warranted, particularly in isolated mucosal disease. Direct immunofluorescence should be performed to rule out oral bullous pemphigoid or pemphigus vulgaris. Histological examination of OLP lesional tissue reveals features similar to those of classic cutaneous LP.
Fig. 4.
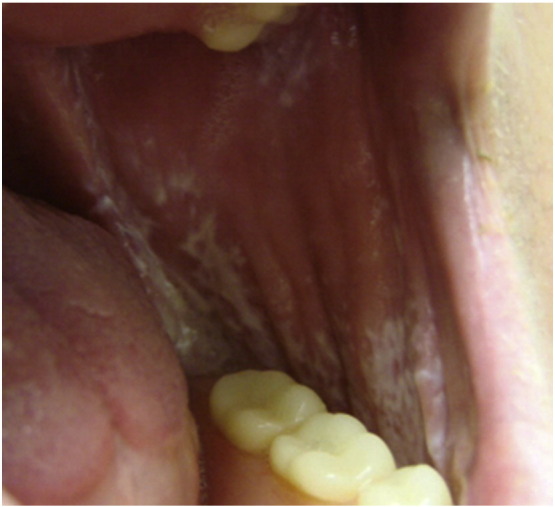
Reticular oral lichen planus. Photo courtesy of Dr. Justin Finch, University of Connecticut Department of Dermatology.
Treatment of OLP in patients varies and depends on the severity of the disease. Primary goals of therapy involve relieving symptoms and monitoring for changes suggestive of dysplasia. Typically, reticular lesions are not treated until they become inflamed, ulcerated, or sore (Le Cleach and Chosidow, 2012, Wagner et al., 2013). While reticular lesions may resolve spontaneously, other forms of OLP are much less likely to do so. First line treatment includes TCS prescribed according to lesion severity (Ismail et al., 2007). Topical immunomodulators can be applied directly or as a rinse. Further, topical retinoids have been used as an alternative choice in treating oral LP. Erosive lesions can be treated with TCS under occlusion. Resistant lesions may require systemic steroid therapy or other systemic immunosuppressants for resolution (Farhi and Dupin, 2010, Ismail et al., 2007).
Nail lichen planus
Nail involvement is a common manifestation of disseminated LP, affecting up to 10% of patients with LP lesions involving other sites (Nakamura et al., 2013). Nail LP may also be the only manifestation of the condition. Nail LP typically appears during the fifth or sixth decades of life and affects genders equally (Tosti et al., 1993).
Typically, nail LP involves the fingernails more than the toenails. Affected nails present with longitudinal ridges, pitting, onychorrhexis, distal splitting, and brown discoloration. Trachyonychia and onycholysis may be apparent. Biopsy of involved tissue shows classic histopathological features of LP. Dermoscopy may aid in evaluation of disease progress and prognosis as changes in the nail matrix, nail bed, and perionychium can be observed with this technique (Nakamura et al., 2013). Early manifestations seen on dermoscopy include pitting of the nail matrix and trachyonychia, while advanced disease often shows chromonychia, lamina fragmentation, onycholysis, and splinter hemorrhage (Nakamura et al., 2013). Differential diagnosis of nail lesions includes psoriasis, onychomycosis, and nail-manifested alopecia areata.
Untreated nail LP may progress to anonychia (Fig. 5), so diagnosis and prompt treatment is important. Conservative first-line therapies include superpotent TCS, often under occlusion, but these are usually unsuccessful. Intralesional steroids are more effective; a single injection of triamcinolone acetonide 10 mg/ml into the proximal nail fold yielded complete recovery within several months and lasted 2 years in one patient (Brauns et al., 2011). Alternatively, systemic steroid therapy with oral prednisone or intramuscular injection of triamcinolone acetonide has provided better outcomes. Unfortunately, recurrence has been reported with all therapies for nail LP (Brauns et al., 2011).
Fig. 5.
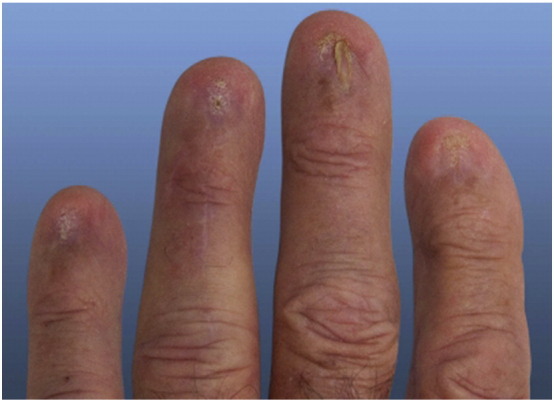
Anonychia in nail lichen planus. Photo courtesy of Dr. Justin Finch, University of Connecticut Department of Dermatology.
Linear lichen planus
Linear LP is a rare variant that affects fewer than 1% of all patients with LP. However, up to 10% of Japanese patients with LP may have the linear form. Gender predominance has not been reported (Batra et al., 2008). While many cases of linear LP may be attributed to a linear pattern of trauma via the Koebner phenomenon, other cases may follow a dermatomal or Blaschkoid pattern (Lehman et al., 2009). Dermatomal or zosteriform patterns may affect skin previously involved in a zoster infection (Pai and Pai, 2013). In linear LP lesions along Blaschko lines, a genetic component predisposing the involved tissue to a lichenoid eruption is probable (Jury and Munro, 2000, Lakshmi et al., 2006).
Clinically, linear LP presents as classic LP papules along a line or band pattern (Fig. 6, Fig. 7). Lesions are usually unilateral, pruritic, and may involve any area of the body (Hartl et al., 1999, Sciallis et al., 2005). Patients may have isolated linear involvement or a combination of a linear presentation with classic lesions elsewhere. Ipsilateral mucosal involvement has also been reported. Differential diagnosis includes inflammatory linear verrucous epidermal nevus, lichen striatus, linear psoriasis, Blaschkitis, and linear Darier–White disease (Horowitz et al., 2013). Histological examination of lesional tissue reveals features identical to classic LP. Treatment is similar to that of classic LP lesions.
Fig. 6.
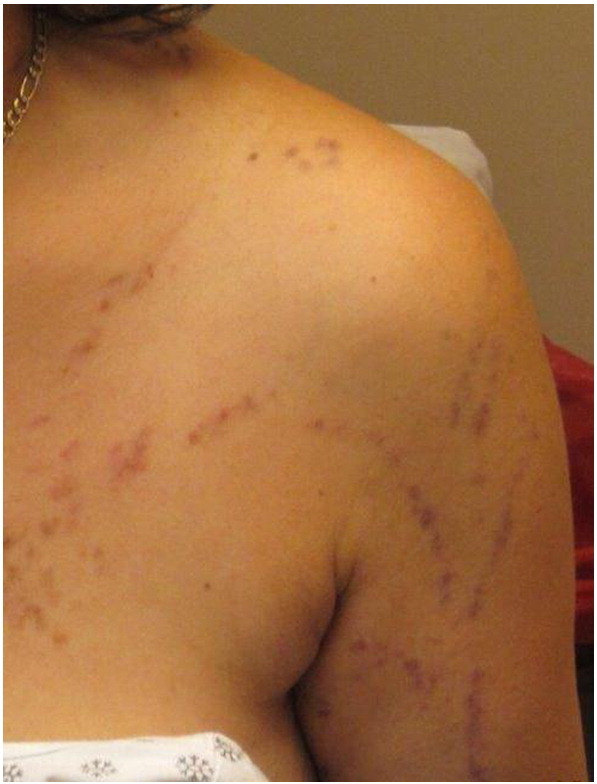
Linear lichen planus. Photo courtesy of Dr. Marti Rothe, University of Connecticut Department of Dermatology.
Fig. 7.
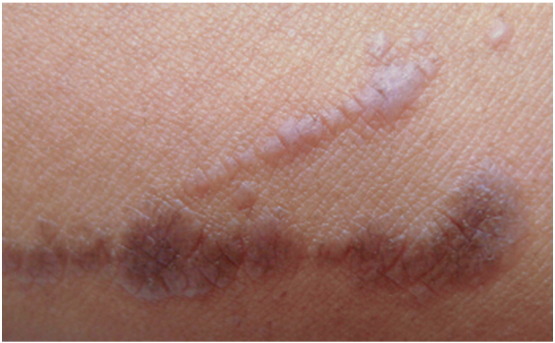
Linear lichen planus. Photo courtesy of Dr. Justin Finch, University of Connecticut Department of Dermatology.
Annular lichen planus
Annular LP is a morphological variant of classic LP. Approximately 3 to 7% of patients with LP have the annular variant, although the true prevalence is likely underestimated (Badri et al., 2011). Reich et al. (2004) observed a male predominance within their study cohort. Specific pathophysiological cause for the annular morphology of these lesions is not well understood, although several theories exist. The ring shape may develop via the convergence of multiple lichenoid papules in a circular shape. Alternatively, other lesions may form as a result of expansion of a papule or plaque with central involution and an advancing raised border.
Annular LP lesions clinically present as red to purple circular macules or plaques with raised borders with or without central atrophy (Iga et al., 2013, Morales-Callaghan et al., 2005) (Fig. 8). They can be single or multiple and can each range between 2 and 8 cm in diameter (Serrao et al., 2008). An increased reporting of lesions that are both atrophic and annular has occurred in the literature, spurring the definition of a new variant, atrophic annular lichen planus. Atrophic lesions are often asymptomatic, although a portion of patients may experience pruritus. Genital lesions, especially of the penis and scrotum, are common and may be the underlying cause of a perceived male predominance (Badri et al., 2011). Lesions also favor the intertriginous zones, including the axillae and groin folds, but can also occur on the lips, trunk, or arms (Holmukhe et al., 2012, Reich et al., 2004). Annular lesions are typically localized, but generalized eruptions have been documented (Lee et al., 2011). Histopathological examination reveals classic features of LP in the active edge of the lesion. Tissue from the center of annular lesions shows a sparser lymphocytic infiltrate and fewer Langerhans cells compared to tissue from the advancing border regions (Yamanaka et al., 2004).
Fig. 8.
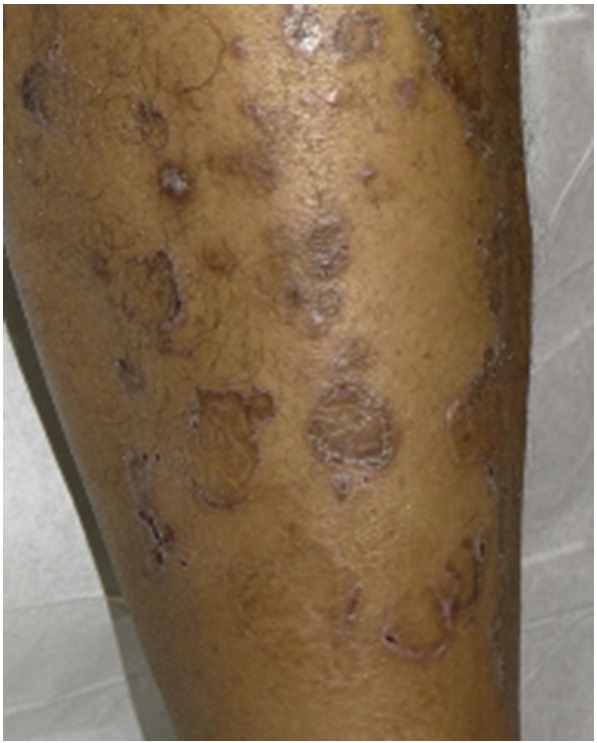
Annular lichen planus. Photo courtesy of Dr. Justin Finch, University of Connecticut Department of Dermatology.
First line treatment of annular LP lesions in the adult patient is similar to that of conventional LP, and involves topical corticosteroids (Badri et al., 2011). Other successful approaches include systemic steroid therapy combined with hydroxychloroquine and dapsone for generalized annular LP and topical calcineurin inhibitors (Lee et al., 2011).
Atrophic lichen planus
Atrophic LP is a rare variant that may occur in areas previously affected by other LP variants. There are very few reports of atrophic LP in recent literature, making prevalence largely unknown. Etiology of the atrophic subtype is not yet elucidated.
Cutaneous lesions of atrophic LP typically arise on skin affected by an earlier LP lesion. They appear as well-demarcated white-bluish or brown papules and plaques, and most often occur after the resolution of annular or ulcerative lesions (Wagner et al., 2013) (Fig. 9). Common sites include the axillae and glans penis, lower extremities, or trunk (Tonsager and Crutchfield, 2004). Lakshmi et al. (2006) reported a case of atrophic LP that also had a linear configuration along the lines of Blaschko. This patient presented with painful, atrophic macules, which progressed to dark brown macules with marked atrophy. Diagnosis of this unusual presentation was confirmed when biopsy showed typical LP features.
Fig. 9.
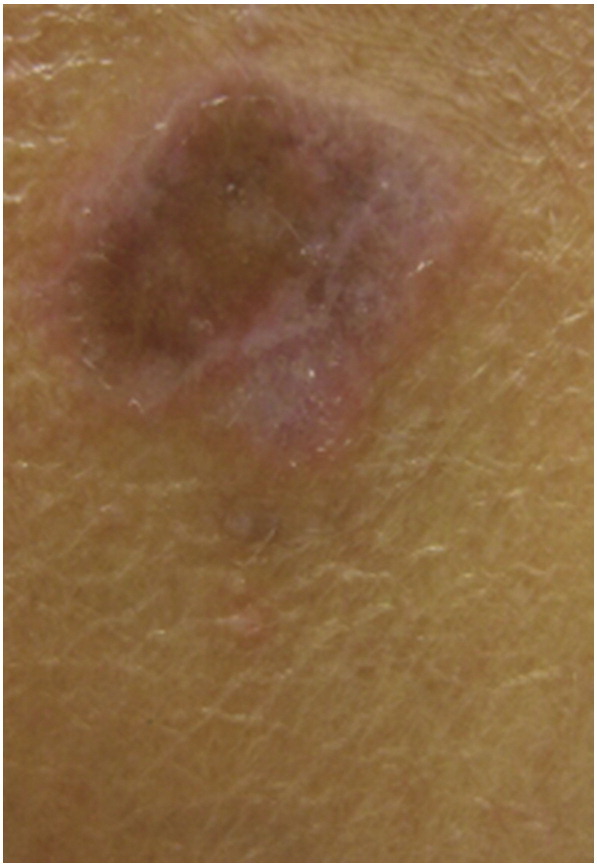
Atrophic lichen planus. Photo courtesy of Dr. Justin Finch, University of Connecticut Department of Dermatology.
First-line treatment is similar to that of other localized lichen planus lesions.
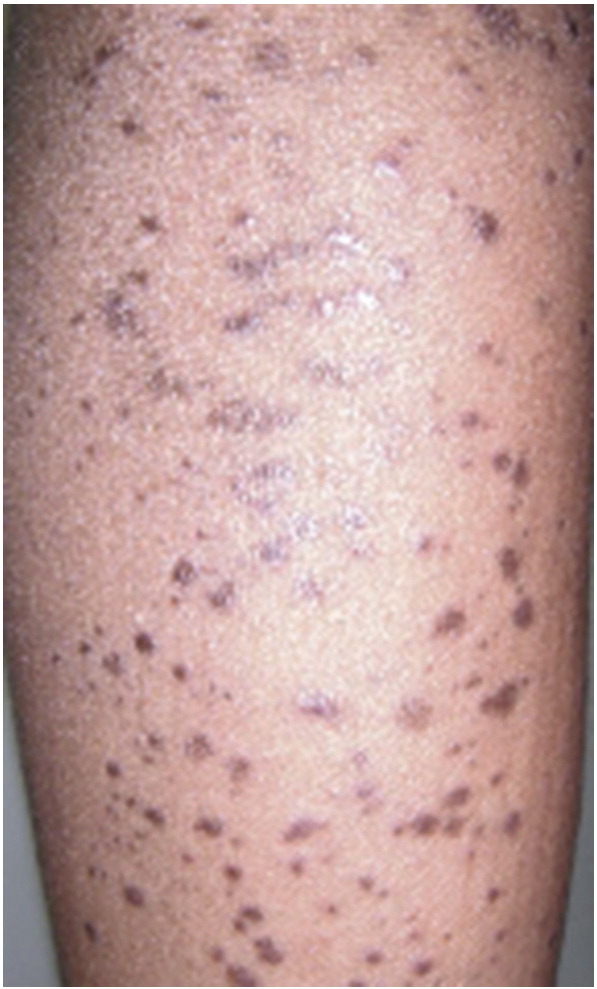
Hypertrophic lichen planus
The hypertrophic variant of LP, also called lichen planus verrucous or lichen planus hypertrophicus, is characterized by thickened papules and plaques and is of unknown prevalence among adults. While exact pathophysiology has not been clarified, eosinophils may play a larger role in hypertrophic LP than in other variants (Alomari and McNiff, 2014). It is likely that the Koebner phenomenon also plays a role in the pathogenesis of these lesions. An association between hypertrophic LP lesions and malignant transformation to squamous cell carcinoma (SCC) has been documented; about 10 cases of squamous cell carcinoma originating from hypertrophic LP lesions have been documented in the literature since 2005. This association may be explained by the chronic inflammation, a known risk factor for SCC, induced by the intense, persistent scratching of these lesions (Krasowska et al., 2012).
Hypertrophic LP lesions typically present as red, yellow-gray, or red-brown papules and plaques, most often on the pretibial area of the lower extremity and the ankles. Lesions can also appear on other areas of the lower limb, the upper limb, trunk, or in a generalized fashion. They are firm to palpation and display a verrucous or hyperkeratotic surface (Fig. 10). They are often extremely pruritic and more chronic in nature than classic LP lesions. Pretibial epidermolysis bullosa may mimic the hypertrophic variant of LP if blistered lesions are not apparent on clinical examination (Apalla et al., 2014). Additionally, severe cases of hypertrophic LP may mimic well-differentiated SCC. Pronounced epidermal hyperplasia and large rete ridges may be present alongside classic histological LP findings in lesional tissue. Pseudocarcinomatous hyperplasia may also be observed.
Fig. 10.
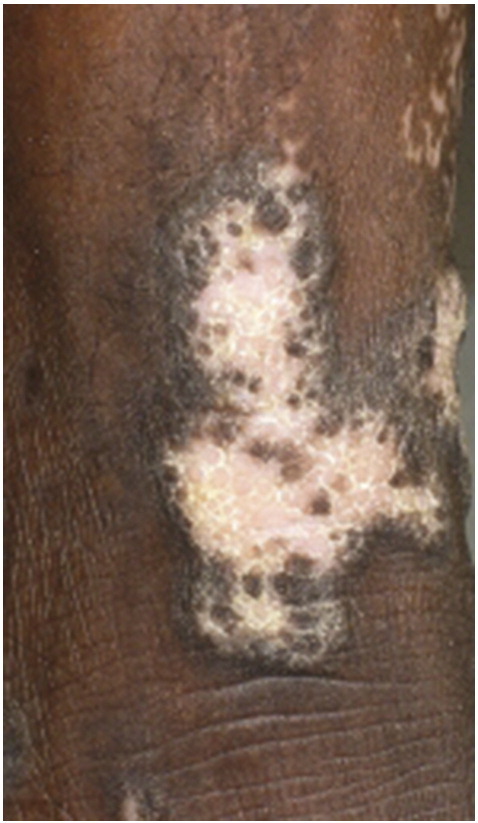
Hypertrophic lichen planus. Photo courtesy of Dr. Justin Finch, University of Connecticut Department of Dermatology.
Treatment of hypertrophic LP lesions falls in line with that of other LP variants, with TCS used as first-line treatments. Successful treatment has been achieved with mycophenolate mofetil, acitretin, and intralesional steroid injections in combination with cryotherapy (Jaime et al., 2011, Nousari et al., 1999, Wagner et al., 2013). Low-dose low-molecular-weight heparin may be effective, but more evidence is needed before use of this treatment becomes commonplace (Rai et al., 2002). Lesions often leave behind hyperpigmentation and scarring after resolution.
Inverse lichen planus
Inverse LP is a variant affecting the intertriginous zones. Prevalence is unknown, as is the exact mechanism behind these site-specific eruptions.
Inverse LP lesions are confined to the intertiginous zones, including the axillae, inguinal creases, gluteal cleft, limb flexures, and submammary region (Lehman et al., 2009). Lesions at these sites may lose their classic morphology, presenting instead as erythematous lesions with poorly defined borders and lichenification (Wagner et al., 2013). Scale may be absent due to the occlusive nature of these locations. Keratotic papules or erosions may be present in individual lesions. Differential diagnosis of inverse LP includes intertriginous manifestions of psoriasis and inverse intertrigo.
Publications on treatment strategies for inverse LP are sparse. TCS are an effective first-line treatment as for other localized cutaneous LP lesions. Additionally, Hoang et al. (2005) found improvement in a patient with inverse LP with tacrolimus ointment.
Eruptive lichen planus
The eruptive variant of LP, also called exanthematous or generalized LP, has rarely been reported in English-language literature in adults. Etiology behind the generalized nature of this form of LP is not well understood. Fleming et al. (2011) reported a case of exanthematous LP triggered by acupuncture, suggesting the influence of external factors in precipitating an outbreak of this form of LP. Interesting, we recently had a case of exanthematous LP at the University of Connecticut.
Clinically, the eruptive form of LP presents as rapidly spreading, disseminated, erythematous, flat-topped, polygonal papules or macules that may become umbilicated and take on a violaceous color. Lesions then resolve, leaving behind hyperpigmented macules (Liu et al., 2013). Lesions may appear over the trunk, all four extremities, and mucosal surfaces (Wagner et al., 2013). Multiple concomitant eruptive lesions may display different morphology, suggesting a chronological evolution of individual papules and macules (Fig. 11).
Fig. 11.
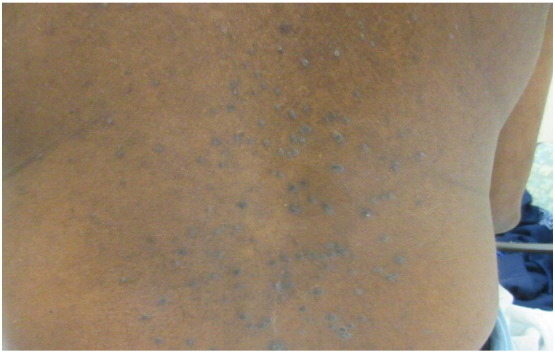
Generalized lichen planus. Photo courtesy of Dr. Marti Rothe, University of Connecticut Department of Dermatology.
First-line treatment of eruptive LP in the adult population is not well documented. Given the involved body surface area, the disseminated nature of this variant likely requires systemic therapy if treatment is desired. A tapered or pulsed systemic corticosteroid therapy alone or in combination with PUVA therapy has been shown to be effective in resolving lesions and preventing new eruptions (Al-Mutairi et al., 2005, Fleming et al., 2011). Similar effects were found with pulsed itraconazole therapy (Khandpur et al., 2009). Kanzaki et al. (1992) found topical steroids, cyclosporine, and systemic griseofulvin to be ineffective, but found success with etretinate. Other forms of UVA and UVB phototherapy may also be helpful additions to systemic steroid therapy (Habib et al., 2005, Wagner et al., 2013). As with most cases of LP, however, spontaneous resolution may eventually occur, as was the case with our patient, who experienced resolution after 8 weeks.
Bullous lichen planus
The bullous form of LP is a rare variant characterized by development of vesico-bullous lesions. Only a few cases have been reported in the literature, making prevalence difficult to estimate. However, the etiology of bullae formation in bullous LP (BLP) is consistent with extensive vacuolar change of the basal cell layer (Gawkrodger et al., 1989). A familial form of BLP has been reported in the literature and seems to be associated with younger age of onset, increased severity, and lengthened duration of the disease compared to that of individuals without a similarly affected first- or second-degree relative (Huang et al., 2007). Familial forms may be inherited in an autosomal dominant pattern and display variable penetrance (Huang et al., 2005).
BLP typically presents as tense bullae on top of typical violaceous, polygonal LP lesions. Many areas can be affected, including the dorsal aspects of the hands and feet as well as the trunk, but the legs are most common site. A bullous lesion superimposed on an asymptomatic annular lichenoid lesion of the glans penis has also been reported (Karthikeyan et al., 2003). BLP may be difficult to distinguish from lichen planus pemphigoides. However, BLP is a subepidermal bulla with degeneration of the epidermal basal layer and classic LP features on histological examination; immunofluorescence in BLP is negative, which is not the case in lichen planus pemphigoides (Gawkrodger et al., 1989).
Treatment of BLP is similar to that of other variants.
Ulcerative lichen planus
The ulcerative or erosive variant of LP may be found on mucosal surfaces, but also occurs on the plantar surface of the feet. Frequency is unknown. Etiology behind the ulcerative nature of this variant is poorly understood, but a potential inductive relationship between metoprolol and erosive LP may exist (Salavastru and Tiplica, 2010).
Ulcerative LP presents clinically as ill-defined, chronic, painful ulcers of the plantar surface. Perilesional skin may be erythematous and scaly. Walking may be impaired, toenails may be lost, and scarring may be present (Salavastru and Tiplica, 2010). Histopathological examination of lesional tissue reveals typical LP features.
Skin grafting to the eroded surface of ulcerative LP lesions has been a therapeutic choice in the past (Wagner et al., 2013). However, less-invasive methods of management have been on the rise. Ulcerative LP lesions are known to be challenging to treat due to resistance to common local and systemic therapies. Topical tacrolimus ointment applied twice daily may lead to near-complete re-epithelialization after 4 weeks in some patients (Salavastru and Tiplica, 2010). Alternatively, medium dose UVA-1 light therapy may produce improvement within several weeks (Mansura et al., 2006). Therapies with documented poor clinical responses include systemic and topical steroids, occlusion under Unna boots, antibiotics, oral retinoids, and topical PUVA therapy.
Lichen planus pigmentosus
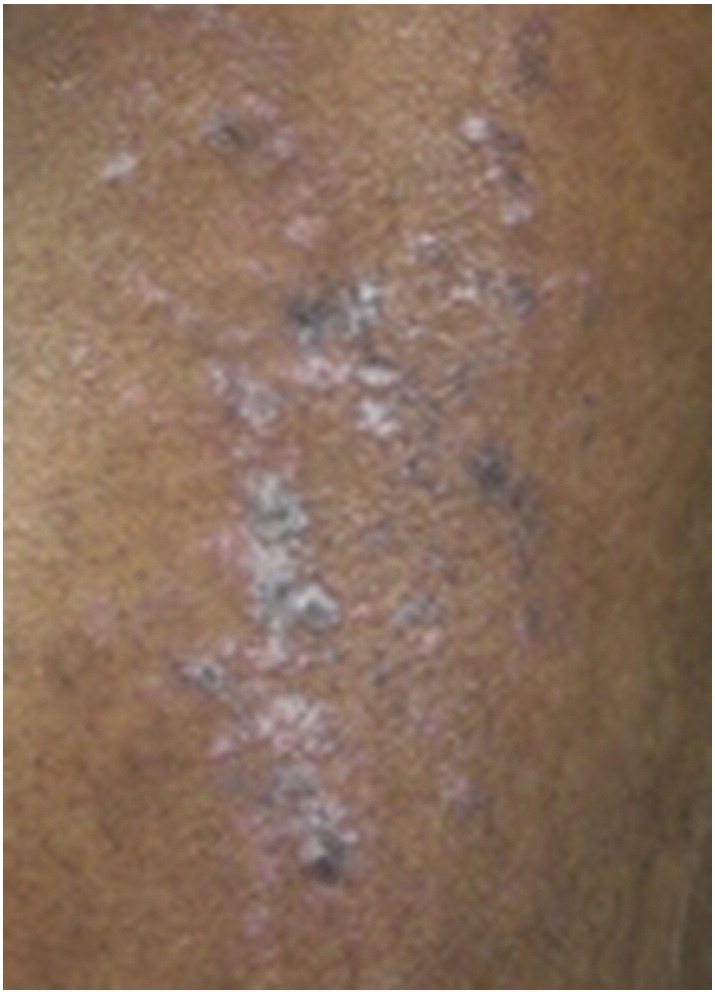
Lichen planus pigmentosus (LPPi) is an uncommon variant that affects all races but seems to favor darker-skinned individuals. It may be more common in Indian and Middle Eastern populations and may occur more frequently in females during the third and fourth decades of life (Bourra and Leila, 2013). Frequency is unknown, as is the exact cause of these lesions. It is possible that pigmentation of these lesions is the result of extensive melanin incontinence. Viral infections (particularly hepatitis C); states of impaired carbohydrate metabolism; drugs; topical application of mustard oil, alma oil, or henna dyes; and sun exposure have all been proposed as triggering factors (Arnold and Cooper, 2011, Torres et al., 2013). Multiple reports of this variant occurring on supravenous skin have suggested that sites of venous dilation or increased intravenous tension may increase skin temperature, which may enhance an antigen immune response (Zhang and Zhu, 2011). This would also explain a reported predominance of pigmented LP lesions in warm, intertriginous sites that has prompted the proposal of a new variant known as lichen planus pigmentosus-inversus (Zhang and Zhu, 2011) (Fig. 12).
Fig. 12.
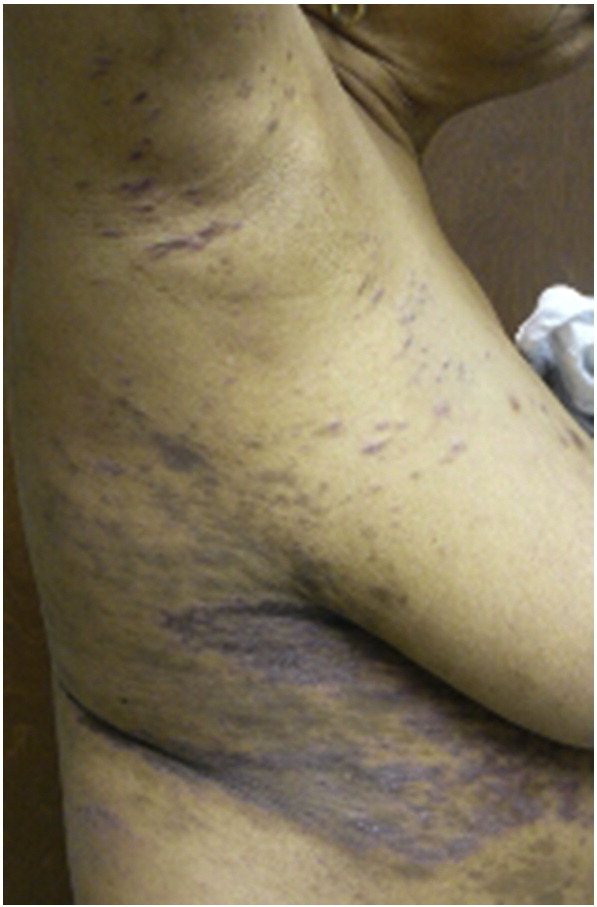
Lichen planus pigmentosus inversus. Photo courtesy of Dr. Justin Finch, University of Connecticut Department of Dermatology.
Typical presentation of LPPi involves hyperpigmented dark brown papules and macules that are often asymptomatic. Lesions usually present on sun-exposed sites including the face, neck, and upper extremities. Flexural area involvement has also been well documented. Pigmentation patterns within lesions are often diffuse, while reticular, blotchy, unilateral linear and perifollicular patterns are less common (Vachiramon et al., 2010). Scalp, nail, or mucosal involvement is rare (Seo et al., 2010). Differential diagnosis includes erythema dyschromicum perstans, post-inflammatory hyperpigmentation, and figurate erythema (Barros, 2013).
LPPi may have a longer clinical course than other variants of LP. First-line therapies are similar to other forms of LP. Successful management has been achieved with TCS and tacrolimus ointment along with acitretin (Rieder et al., 2013). Poor response to chloroquine has been reported (Bourra and Leila, 2013). Phototherapy should be avoided due to increased risk of pigmentation.
Lichen planopilaris
Lichen planopilaris (LPP) is a morphological variant of LP involving the hair follicles that has been classified as a primary lymphocytic cicatricial alopecia. It occurs more frequently in Caucasian and Indian populations, with lower incidence in Asian populations. Affected female to male ratio is 1.8:1. Most patients present between the second and seventh decades of life (Lehman et al., 2009, Kang et al., 2008). Exact etiology is poorly understood but is likely related to an inflammatory response mediated by T lymphocytes targeting follicular antigens. It is possible that infection, metal exposure, stress, and other factors may act as sensitizers or triggers for the condition.
Clinically, patients with LPP present with whitish atrophic or scarring patches on the scalp that may be accompanied by complaints of increased hair shedding, itching, scaling, burning, and tenderness of the scalp (Fig. 13). These subjective symptoms may worsen with exposure to ultraviolet light, scalp irritation, sweating, and stress. Anagen hairs may be pulled easily from active lesions. Lesions may be single or multiple and commonly involve the vertex and parietal area. Three groups of LPP exist: classic, frontal fibrosing alopecia, and Graham-Little-Piccardi-Lassueur syndrome. Classic LPP presents with scalp lesions and occasional extracranial LP lesions. Frontal fibrosing alopecia presents with progressive band-like scarring and hair loss from the frontal hairline and mostly affects middle-aged women. Graham-Little-Piccardi-Lassueur syndrome involves a triad of cicatricial alopecia of the scalp as well as lichen planus of the skin with widespread follicular papules and a non-scarring hair loss of the axillary and pubic area (Kang et al., 2008). Differential diagnosis of LPP includes other scarring alopecias such as discoid lupus erythematosus and central centrifugal cicatricial alopecia. Further, early LPP may be confused with seborrheic dermatitis. Histopathological examination of early, active lesions reveals a lichenoid lymphocytic infiltrate affecting the infundibulum and isthmus. The basement membrane zone, sebaceous glands, and root sheaths are eventually lost and are replaced with fibrosis and scarring (Tandon et al., 2008). Histopathological examination of advanced lesions often reveals thick fibrous tracts that have replaced destroyed hair follicles (Cevasco et al., 2007).
Fig. 13.
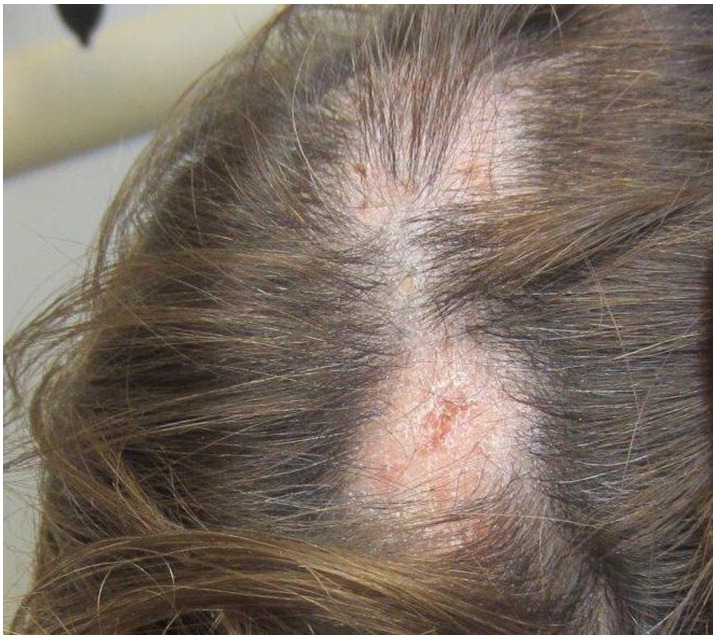
Lichen planopilaris. Photo courtesy of Dr. Marti Rothe, University of Connecticut Department of Dermatology.
First-line treatment for mild to moderate LPP involving less than 10% of the scalp is often with superpotent TCS or intralesional corticosteroid injections. Tetracycline derivatives or topical tacrolimus have shown varied effectiveness (Blazek and Megahed, 2008, Cevasco et al., 2007, Kang et al., 2008). Severe, rapidly progressing disease or patients with severe symptoms may require oral corticosteroids. Oral steroid therapy can also be used as a bridge therapy until other agents have reached maximal effectiveness (Kang et al., 2008). Another approach to treatment in patients with extensive scalp involvement and lesions resistant to steroid therapy is with hydroxychloroquine, which may show improvement within several weeks but may take up to 6 to 12 months until maximally effective (Kang et al., 2008, Samrao et al., 2010). Further refractory cases of lichen planopilaris may be treated with immunosuppressive agents including cyclosporine and mycophenolate mofetil. Third-line therapies such as systemic retinoids, griseofulvin, thalidomide, dapsone, and minoxidil are still controversial (Kang et al., 2008). Some success has been documented with the use of peroxisome proliferator-activated receptor γ agonists like pioglitazone (Mirmirani and Karnik, 2009). Finally, a combination of dutasteride and pimecrolimus was reported effective in a patient with the frontal fibrosing variant of LPP (Perez-Rodriguez et al., 2013).
Vulvovaginal lichen planus
Vulvovaginal LP is an uncommon variant that involves the vulva and vagina. This disease seems to largely affect Caucasian women of perimenopausal age. Most patients present in the sixth decade of life (Anderson et al., 2002). Incidence and etiology have not been established. An association of vulvovaginal lesions and development of vulval intraepithelial neoplasia may exist, but remains controversial (Anderson et al., 2002, Neill and Lewis, 2008). Further, vulvovaginal LP may occasionally be referred to vulvovaginal-gingival syndrome as patients with vulvovaginal lesions often also have oral lesions, especially those affecting the gingival tissue.
A characteristic feature of the vulvovaginal variant of LP is a chronic course with unexplained exacerbations, improvements, and remissions. Women affected by vulvovaginal LP may present to a number of providers complaining of genital irritation, itching, burning, soreness, and dyspareunia. These symptoms may be accompanied by abnormal, often purulent discharge if the lesions involve the vagina. On physical examination, findings may vary but usually suggest inflamed, erythematous, and/or eroded epithelium. Eroded epithelium may transform into a reticulated white-gray lacy pattern. The disease may progress to vaginal adhesion, stenosis, or obliteration, making early diagnosis crucial (Sobel, 2002). Differential diagnosis includes lichen sclerosis, autoimmune vesicobullous diseases, plasma cell vulvitis, Behcets disease, and desquamative inflammatory vaginitis. Classic histological features of LP may be obscured by the chronic and erosive nature of vulvovaginal lesions, but hyperkeratosis and/or parakeratosis with varying degrees of epidermal thinning and ulceration and lichenoid lymphocytic infiltrate may be appreciated (Sobel, 2002).
Treatment of vulvovaginal LP is often challenging secondary to the chronic nature of the disease and an apparent delay in diagnosis for many women. There is a distinct need for randomized controlled trials on various therapies in the management of vulvovaginal LP. Recently, Bradford and Fischer (2013) demonstrated improvements in disease course with initial aggressive therapy involving oral prednisolone, with or without additional TCS application, and maintenance therapy with weekly low-dose methotrexate when topical therapy was not adequate. Alternatively, several studies have suggested that steroid vaginal suppositories may be useful in controlling vulvovaginal LP lesions (Anderson et al., 2002, Sobel, 2002). Topical calcineurin inhibitors may be useful in some patients, but may increase risk of neoplastic transformation in genital tissue (Neill and Lewis, 2008). Other therapies reported anecdotally in the literature include cyclosporine, dapsone, griseofulvin, retinoid gel, thalidomide, antibiotics, hydroxychloroquine, and other immunosuppressive agents (Sobel, 2002).
Actinic lichen planus
Actinic LP, also called lichen planus subtropicus or lichen planus actinicus, is a rare variant that affects sun-exposed areas of the skin. Reports of actinic LP are more common in darker-skinned African, Middle Eastern, and Indian populations, while very few cases have been reported in Caucasians (Dekio et al., 2010). Eruptions occur more often during spring and summer, with improvement or remission during the winter months. Actinic LP is more often reported in middle-aged patients and may be more common in females than males (Prakash et al., 2013). Exact pathogenesis is unknown but sun exposure is clearly a major precipitating factor. There are likely underlying genetic, infectious, environmental, or hormonal factors that may predispose individuals to this subtype of LP.
Clinically, actinic LP can present in three forms: annular, pigmented, and dyschromic. The most common form, annular actinic LP, is characterized by erythematous brownish plaques in an annular configuration with or without atrophy. Pigmented forms present as hypermelanotic patches with a melasma-like appearance. Dyschromic-type actinic LP lesions are the rarest and are characterized by whitish pinhead and coalescent papules (Mebazaa et al., 2010) (Fig. 14). Frequently affected sites include the face, especially the forehead, cheeks and lips; the upper chest; the extensor surface of the distal forearms; and the dorsal surface of the hands. Actinic lesions are commonly asymptomatic and may resemble actinic keratosis. Diagnosis is typically made based on distribution in sun-exposed areas in combination with histological findings consistent with those of classic LP.
Fig. 14.
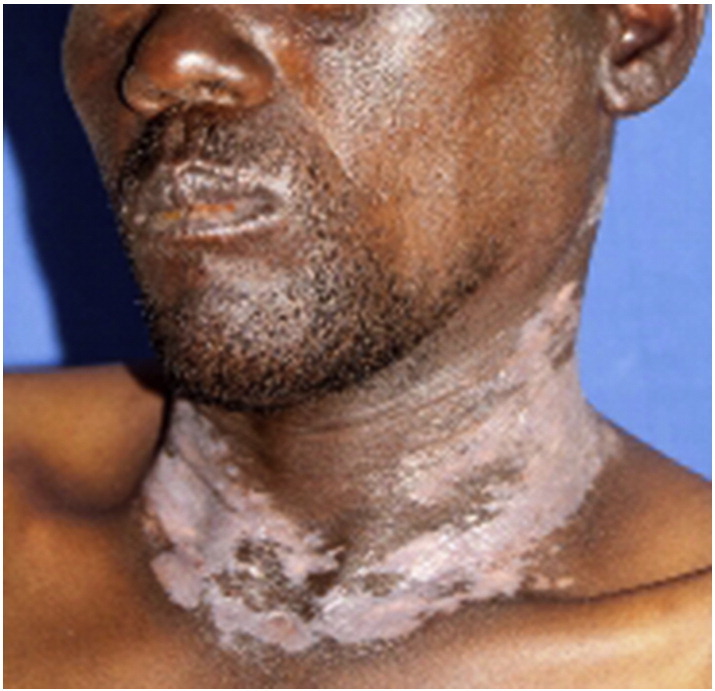
Actinic lichen planus. Photo courtesy of Dr. Justin Finch, University of Connecticut Department of Dermatology.
Treatment for actinic LP lesions in the adult patient can vary. TCS, a common therapeutic choice, have been shown to be effective in improving symptoms but are associated with relapses with subsequent sun exposure (Mebazaa et al., 2010). Improvement and prolonged remission of lesions have been reported with topical pimecrolimus cream, cyclosporine, or intralesional steroid injections in combination with sunscreen (Ezzedine et al., 2009, Gallo et al., 2008). Varying results have been found with oral retinoids, systemic steroid therapy, bismuth or arsenic compounds, hydroxychloroquine, and intense pulsed light (Mebazaa et al., 2010, Santos-Juanes et al., 2010). Phototherapy should be avoided in these patients as lesions are characteristically associated with light exposure (Wagner et al., 2013). Sun avoidance is an essential part of any therapeutic regimen targeted at improving actinic LP lesions.
Lichen planus-lupus erythematosus overlap syndrome
Lichen planus-lupus erythematosus overlap syndrome is a rare variant that displays features of both LP and lupus erythematosus in the same patient or in the same lesion in a single patient. Fewer than 50 cases have been reported in the literature before 2011 (Sekar et al., 2011). There is much controversy over whether these two diseases actually overlap (necessitating the distinction of this overlap syndrome) or if reports are simply coexistent episodes of these two diseases in the same patient. Not surprisingly, no etiology has been clarified. An autoimmune cause is likely due to the role of autoimmunity in both lupus erythematosus and LP.
Lesions may present clinically with features of lupus erythematosus, LP, or both. The distal arms, legs, face, and trunk may be involved. Palmoplantar involvement may be characteristic of this syndrome (Fig. 15). Cases have been reported in which patients have separate lesions consistent with each disease; Demirci et al. (2011) reported a man who had a malar butterfly rash and erythema of the back, consistent with lupus erythematosus, and classic LP lesions on the extremities. Alternatively, Nagao and Chen (2006) reported a case of genuine overlap within a single lesion in a female patient. Histological examination, again, may reveal characteristics of each disease or both simultaneously, which may complicate diagnosis. Direct immunofluorescence may be essential in diagnosing this overlap syndrome (Camisa et al., 1984). While some lesions still may only show features of one disease, some may show granular deposits of immunoglobulins, complement components and fibrin along the dermal epidermal junction, and clusters of IgM-staining colloid bodies, which would be suggestive of both lupus erythematosus and lichen planus in the same tissue specimen (Nagao and Chen, 2006).
Fig. 15.
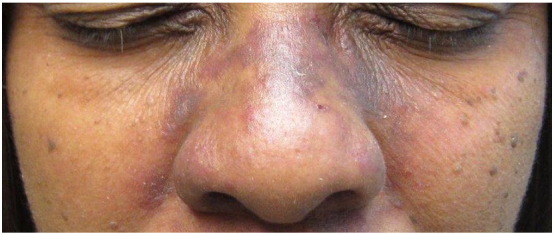
Lichen planus/ lupus erythematosus overlap. Photo courtesy of Dr. Marti Rothe, Univeristy of Connecticut Department of Dermatology.
Treatment of this variant often requires systemic therapy. However, remarkable clinical improvement has been reported with high-potency TCS and acitretin (Demirci et al., 2011, Lospinoso et al., 2013). Topical tacrolimus ointment produced a moderate effect without eradicating the lesions in the patient with genuine overlap discussed previously (Nagao and Chen, 2006). Cyclosporine has also been reported to be effective (Demirci et al., 2011).
Lichen planus pemphigoides
Lichen planus pemphigoides (LPPe) is a rare autoimmune subepidermal blistering dermatosis that may be a unique variant of LP or a heterogeneous blistering response to various antigens exposed by multiple injuries to the basal membrane zone. It is characterized by clinical, histological, and immunopathological features of both classic LP and bullous pemphigoid (Lehman et al., 2009). Exact prevalence is not clear, but a male predominance has been suggested. Patients commonly present in the fourth or fifth decade of life and all races can be affected. Etiology has not been fully elucidated; however, LPPe is likely the result of an autoimmune response to hemidesmosomal- or keratinocyte-derived antigens that have been exposed as a result of basal keratinocyte apoptosis or injury (Hirayama et al., 2012, Kasperkiewicz et al., 2012). LPPe eruption may be sporadic, a consequence of adverse drug reaction (ACE inhibitors, cinnarizine, or simvastatin), or a complication of PUVA therapy. LPPe has also been linked to Castleman’s disease and various neoplasms, including retroperitoneal round-cell liposarcoma, chronic lymphocytic leukemia, and other soft-tissue tumors (Anand et al., 2011).
LPPe lesions characteristically involve tense bullae affecting both uninvolved skin and skin affected by prior or current lichenoid eruptions. Lesions commonly involve the extremities but may occur anywhere, including the oral mucosa. Histological examination of lesional tissue reveals characteristics of both lichen planus and bullous pemphigoid. On direct immunofluorescence, fibrinogen deposition at the dermal–epidermal junction and cytoid bodies are seen, consistent with lichen planus, while probing for IgG and C3 is positive at the basement membrane zone, which is consistent with bullous pemphigoid (Anand et al., 2011).
One approach to the treatment of LPPe is to treat the existing LP and avoid further immunostimulation. Alternatively, the lesions may be treated as bullous pemphigoid lesions (Kasperkiewicz et al., 2012). Treatment of LPPe with topical steroids may be effective, while some cases require systemic steroid therapy. Cyclosporine seems to be a beneficial addition to systemic steroid therapy in recalcitrant cases. Additionally, tetracycline, nicotinamide, isotretinoin, dapsone, and other immunosuppressive drugs have produced varying results (Anand et al., 2011). Due to the association of LPP eruptions with Castleman’s disease, paraneoplastic pemphigus, and several malignancies, patients with an LPPe diagnosis should undergo assessment for unknown neoplasms (Hirayama et al., 2012).
Conclusion
Lichen planus is an inflammatory skin disease with characteristic clinical and histopathological findings. In addition to classic LP, a myriad of LP variants exist, including oral, nail, linear, annular, atrophic, hypertrophic, inverse, eruptive, bullous, ulcerative, LP pigmentosus, lichen planopilaris, vulvovaginal, actinic, LP-lupus erythematosus overlap syndrome, and LP pemphigoides. The pruritic, polygonal, violaceous, flat-topped papules and plaques of classic LP are the most common presentation of the disease, but morphology and location vary greatly among the variants. However, histopathological findings among the variants are largely consistent. Therefore, while clinical examination may be sufficient for diagnosis in some cases, histological examination is often valuable in confirming diagnosis of LP variants. Management of classic LP and its variants is mostly consistent, with topical TCS as a first-line therapy among a diverse array of treatment options that have been reported in the literature with varying degrees of success. Ultimately, familiarity with the characteristics of LP and its variants is essential in achieving timely recognition and effective management.
Acknowledgments
The authors would like to thank Dr. Marti Rothe at University of Connecticut, who generously donated the funding for publication for this article.
Footnotes
Conflict of interest: Michael Payette is a consultant for Amgen and Lilly.
References
- Al-Mutairi N., Joshi A., Zaki A., Sharma A.K., Nour-Eldin O. Acute generalized lichen planus treated with weekly betamethasone 5-mg oral mini-pulse therapy. J Drugs Dermatol. 2005;4(2):218–220. [PubMed] [Google Scholar]
- Alomari A., McNiff J.M. The significance of eosinophils in hypertrophic lichen planus. J Cutan Pathol. 2014;41(4):347–352. doi: 10.1111/cup.12275. [DOI] [PubMed] [Google Scholar]
- Anand D., Bernardin R., Rubin A.I. Blisters and plaques on the extremities. What is your diagnosis? Lichen planus pemphigoides. Int J Dermatol. 2011;50(2):147–149. doi: 10.1111/j.1365-4632.2010.04722.x. [DOI] [PubMed] [Google Scholar]
- Anderson M., Kutzner S., Kaufman R.H. Treatment of vulvovaginal lichen planus with vaginal hydrocortisone suppositories. Obstet Gynecol. 2002;100(2):359–362. doi: 10.1016/s0029-7844(02)02117-8. [DOI] [PubMed] [Google Scholar]
- Apalla Z., Lallas A., Karakyriou E., Karatolias A., Sotiriou E., Chaidemenos G. Pretibial epidermolysis bullosa mimicking hypertrophic lichen planus. Int J Dermatol. 2014;53(3):e197–e199. doi: 10.1111/j.1365-4632.2012.05778.x. [DOI] [PubMed] [Google Scholar]
- Arnold S., Cooper S. 2011. Lichen planus pigmentosus. [Internet] [Accessed, cited 2014 January 31 http://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=EN&Expert=254463] [Google Scholar]
- Badri T., Kenani N., Benmously R., Debbiche A., Mokhtar I., Fenniche S. Isolated genital annular lichen planus. Acta Dermatovenerol Alp Panonica Adriat. 2011;20(1):31–33. [PubMed] [Google Scholar]
- Balasubramaniam P., Ogboli M., Moss C. Lichen planus in children: review of 26 cases. Clin Exp Dermatol. 2008;33(4):457–459. doi: 10.1111/j.1365-2230.2008.02694.x. [DOI] [PubMed] [Google Scholar]
- Barros Hugo Rocha, Paes de Almeida José Roberto, Mattos e Dinato Sandra Lopes, Sementilli Angelo, Ney Romit. Lichen planus pigmentosus inversus. Anais Brasileiros de Dermatologia. 2013;88(6, Suppl 1):146–149. doi: 10.1590/abd1806-4841.20132599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra P., Wang N., Kamino H., Possick P. Linear lichen planus. Dermatol Online J. 2008;14(10):16. [PubMed] [Google Scholar]
- Bilgili S.G., Karadag A.S., Ozkol H.U., Calka O., Akdeniz N. The prevalence of skin diseases among the geriatric patients in eastern Turkey. J Pak Med Assoc. 2012;62(6):535–539. [PubMed] [Google Scholar]
- Blazek C., Megahed M. Lichen planopilaris. Successful treatment with tacrolimus. Hautarzt. 2008;59(11):874–877. doi: 10.1007/s00105-008-1650-8. [DOI] [PubMed] [Google Scholar]
- Bourra H., Leila B. Lichen planus pigmentosus. Pan Afr Med J. 2013;15:55. doi: 10.11604/pamj.2013.15.55.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford J., Fischer G. Management of vulvovaginal lichen planus: a new approach. J Low Genit Tract Dis. 2013;17(1):28–32. doi: 10.1097/LGT.0b013e318258bf5b. [DOI] [PubMed] [Google Scholar]
- Brauns B., Stahl M., Schon M.P., Zutt M. Intralesional steroid injection alleviates nail lichen planus. Int J Dermatol. 2011;50(5):626–627. doi: 10.1111/j.1365-4632.2010.04786.x. [DOI] [PubMed] [Google Scholar]
- Camisa C., Neff J.C., Olsen R.G. Use of indirect immunofluorescence in the lupus erythematosus/lichen planus overlap syndrome: an additional diagnostic clue. J Am Acad Dermatol. 1984;11(6):1050–1059. doi: 10.1016/s0190-9622(84)70258-1. [DOI] [PubMed] [Google Scholar]
- Cevasco N.C., Bergfeld W.F., Remzi B.K., de Knott H.R. A case-series of 29 patients with lichen planopilaris: the Cleveland Clinic Foundation experience on evaluation, diagnosis, and treatment. J Am Acad Dermatol. 2007;57(1):47–53. doi: 10.1016/j.jaad.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Dekio I., Matsuki S., Furumura M., Morita E., Morita A. Actinic lichen planus in a Japanese man: first case in the east asian population. Photodermatol Photoimmunol Photomed. 2010;26(6):333–335. doi: 10.1111/j.1600-0781.2010.00548.x. [DOI] [PubMed] [Google Scholar]
- Demirci G.T., Altunay I.K., Sarikaya S., Sakiz D. Lupus erythematosus and lichen planus overlap syndrome: a case report with a rapid response to topical corticosteroid therapy. Dermatol Rep. 2011;3(3):e48. doi: 10.4081/dr.2011.e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRossi S.S., Ciarrocca K.N. Lichen planus, lichenoid drug reactions, and lichenoid mucositis. Dent Clin North Am. 2005;49(1):77–89. doi: 10.1016/j.cden.2004.08.004. [viii] [DOI] [PubMed] [Google Scholar]
- Ezzedine K., Simonart T., Vereecken P., Heenen M. Facial actinic lichen planus following the Blaschko's lines: successful treatment with topical 0.1% pimecrolimus cream. J Eur Acad Dermatol Venereol. 2009;23(4):458–459. doi: 10.1111/j.1468-3083.2008.02903.x. [DOI] [PubMed] [Google Scholar]
- Farhi D., Dupin N. Pathophysiology, etiologic factors, and clinical management of oral lichen planus, part I: facts and controversies. Clin Dermatol. 2010;28(1):100–108. doi: 10.1016/j.clindermatol.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Fleming J., Diaz-Cano S., Higgins E. Eruptive lichen planus triggered by acupuncture. Arch Dermatol. 2011;147(3):361–362. doi: 10.1001/archdermatol.2011.29. [DOI] [PubMed] [Google Scholar]
- Gallo L., Ayala F., Ayala F. Relapsing lichen actinicus successfully treated with cyclosporin. J Eur Acad Dermatol Venereol. 2008;22(3):370–371. doi: 10.1111/j.1468-3083.2007.02325.x. [DOI] [PubMed] [Google Scholar]
- Gawkrodger D.J., Stavropoulos P.G., McLaren K.M., Buxton P.K. Bullous lichen planus and lichen planus pemphigoides: clinico-pathological comparisons. Clin Exp Dermatol. 1989;14(2):150–153. doi: 10.1111/j.1365-2230.1989.tb00914.x. [DOI] [PubMed] [Google Scholar]
- Habib F., Stoebner P.E., Picot E., Peyron J.L., Meynadier J., Meunier L. Narrow band UVB phototherapy in the treatment of widespread lichen planus. Ann Dermatol Venereol. 2005;132(1):17–20. doi: 10.1016/s0151-9638(05)79189-4. [DOI] [PubMed] [Google Scholar]
- Handa S., Sahoo B. Childhood lichen planus: a study of 87 cases. Int J Dermatol. 2002;41(7):423–427. doi: 10.1046/j.1365-4362.2002.01522.x. [DOI] [PubMed] [Google Scholar]
- Hartl C., Steen K.H., Wegner H., Seifert H.W., Bieber T. Unilateral linear lichen planus with mucous membrane involvement. Acta Derm Venereol. 1999;79(2):145–146. doi: 10.1080/000155599750011390. [DOI] [PubMed] [Google Scholar]
- Hirayama A., Maruta C., Santi C., Aoki V. Lichen planus pemphigoides: case reports and review of the literature. J Am Acad Dermatol. 2012;66(4):AB104. [Google Scholar]
- Hiremath S.K., Kale A.D., Charantimath S. Oral lichenoid lesions: clinico-pathological mimicry and its diagnostic implications. Indian J Dent Res. 2011;22(6):827–834. doi: 10.4103/0970-9290.94679. [DOI] [PubMed] [Google Scholar]
- Hoang J., Malone J., Callen J. Inverse lichen planus: an unusual morphologic variant of a classic papulosquamous dermatosis. J Am Acad Dermatol. 2005;52(3):64. [Google Scholar]
- Holmukhe S.F., Gutte R.M., Sirur S. Letter: Isolated annular lichen planus of lower lip. Dermatol Online J. 2012;18(2):15. [PubMed] [Google Scholar]
- Horowitz M.R., Vidal Mde L., Resende M.O., Teixeira M.A., Cavalcanti S.M., Alencar E.R. Linear lichen planus in children—case report. An Bras Dermatol. 2013;88(6 Suppl 1):139–142. doi: 10.1590/abd1806-4841.20131971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Chen S., Liu Z., Tao J., Wang C., Zhou Y. Familial bullous lichen planus (FBLP): pedigree analysis and clinical characteristics. J Cutan Med Surg. 2005;9(5):217–222. doi: 10.1007/s10227-005-0146-8. [DOI] [PubMed] [Google Scholar]
- Huang C., Yan X., Yang L., Zhang J., Tian J., Li J. A retrospective and comparative study of familial and non-familial bullous lichen planus. J Huazhong Univ Sci Technolog Med Sci. 2007;27(3):336–338. doi: 10.1007/s11596-007-0331-7. [DOI] [PubMed] [Google Scholar]
- Iga N., Sakurai K., Murata T., Ehara M., Tanaka M., Honda T. Wickham's striae presented with whitish ring-form on annular lichen planus. J Dermatol. 2013;40(12):1060–1061. doi: 10.1111/1346-8138.12345. [DOI] [PubMed] [Google Scholar]
- Ismail S.B., Kumar S.K., Zain R.B. Oral lichen planus and lichenoid reactions: etiopathogenesis, diagnosis, management and malignant transformation. J Oral Sci. 2007;49(2):89–106. doi: 10.2334/josnusd.49.89. [DOI] [PubMed] [Google Scholar]
- Jaime T.J., Jaime T.J., Guaraldi Bde M., Melo D.F., Jeunon T., Lerer C. Disseminated hypertrophic lichen planus: relevant response to acitretin. An Bras Dermatol. 2011;86(4 Suppl 1):S96–S99. doi: 10.1590/s0365-05962011000700025. [DOI] [PubMed] [Google Scholar]
- Jury C.S., Munro C.S. Linear lichen planus related to hepatitis C infection? Br J Dermatol. 2000;142(4):836–837. doi: 10.1046/j.1365-2133.2000.03450.x. [DOI] [PubMed] [Google Scholar]
- Kang H., Alzolibani A.A., Otberg N., Shapiro J. Lichen planopilaris. Dermatol Ther. 2008;21(4):249–256. doi: 10.1111/j.1529-8019.2008.00206.x. [DOI] [PubMed] [Google Scholar]
- Kanwar A.J., De D. Lichen planus in childhood: report of 100 cases. Clin Exp Dermatol. 2010;35(3):257–262. doi: 10.1111/j.1365-2230.2009.03613.x. [DOI] [PubMed] [Google Scholar]
- Kanzaki T., Otake N., Nagai M. Eruptive lichen planus. J Dermatol. 1992;19(4):234–237. doi: 10.1111/j.1346-8138.1992.tb03214.x. [DOI] [PubMed] [Google Scholar]
- Karthikeyan K., Jeevankumar B., Thappa D.M. Bullous lichen planus of the glans penis. Dermatol Online J. 2003;9(5):31. [PubMed] [Google Scholar]
- Kasperkiewicz M., Zillikens D., Schmidt E. Pemphigoid diseases: pathogenesis, diagnosis, and treatment. Autoimmunity. 2012;45(1):55–70. doi: 10.3109/08916934.2011.606447. [DOI] [PubMed] [Google Scholar]
- Khandpur S., Sugandhan S., Sharma V.K. Pulsed itraconazole therapy in eruptive lichen planus. J Eur Acad Dermatol Venereol. 2009;23(1):98–101. doi: 10.1111/j.1468-3083.2008.02743.x. [DOI] [PubMed] [Google Scholar]
- Krasowska D., Kozlowicz K., Kowal M., Kurylcio A., Budzynska-Wlodarczyk J., Polkowski W. Twice malignant transformation of hypertrophic lichen planus. Ann Agric Environ Med. 2012;19(4):787–789. [PubMed] [Google Scholar]
- Kumar V., Garg B.R., Baruah M.C., Vasireddi S.S. Childhood lichen planus (LP) J Dermatol. 1993;20(3):175–177. doi: 10.1111/j.1346-8138.1993.tb03854.x. [DOI] [PubMed] [Google Scholar]
- Kyriakis K.P., Terzoudi S., Palamaras I., Michailides C., Emmanuelidis S., Pagana G. Sex and age distribution of patients with lichen planus. J Eur Acad Dermatol Venereol. 2006;20(5):625–626. doi: 10.1111/j.1468-3083.2006.01513.x. [DOI] [PubMed] [Google Scholar]
- Lakshmi C., Divakaran J., Sivaraman A., Srinivas C. Painful linear atrophic lichen planus along lines of Blaschko. Indian J Dermatol. 2006;51(1):42–43. [Google Scholar]
- Le Cleach L., Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366(8):723–732. doi: 10.1056/NEJMcp1103641. [DOI] [PubMed] [Google Scholar]
- Lee J.B., Wi H.S., Han J.H., Kim S.J., Yung S.J. A case of generalized annular lichen planus. J Am Acad Dermatol. 2011;64(2, Supplement 1):AB162. [Google Scholar]
- Lehman J.S., Tollefson M.M., Gibson L.E. Lichen planus. Int J Dermatol. 2009;48(7):682–694. doi: 10.1111/j.1365-4632.2009.04062.x. [DOI] [PubMed] [Google Scholar]
- Liu K.C., Lee J.Y., Hsu M.M., Hsu C.K. The evolution of clinicopathologic features in eruptive lichen planus: a case report and review of literature. Dermatol Online J. 2013;19(1):8. [PubMed] [Google Scholar]
- Lospinoso D.J., Fernelius C., Edhegard K.D., Finger D.R., Arora N.S. Lupus erythematosus/lichen planus overlap syndrome: successful treatment with acitretin. Lupus. 2013;22(8):851–854. doi: 10.1177/0961203313492243. [DOI] [PubMed] [Google Scholar]
- Luis-Montoya P., Dominguez-Soto L., Vega-Memije E. Lichen planus in 24 children with review of the literature. Pediatr Dermatol. 2005;22(4):295–298. doi: 10.1111/j.1525-1470.2005.22402.x. [DOI] [PubMed] [Google Scholar]
- Mansura A., Alkalay R., Slodownik D., Ingber A., Ruzicka T., Enk C.D. Ultraviolet A-1 as a treatment for ulcerative lichen planus of the feet. Photodermatol Photoimmunol Photomed. 2006;22(3):164–165. doi: 10.1111/j.1600-0781.2006.00200.x. [DOI] [PubMed] [Google Scholar]
- Mebazaa A., Denguezli M., Ghariani N., Sriha B., Belajouza C., Nouira R. Actinic lichen planus of unusual presentation. Acta Dermatovenerol Alp Panonica Adriat. 2010;19(2):31–33. [PubMed] [Google Scholar]
- Mirmirani P., Karnik P. Lichen planopilaris treated with a peroxisome proliferator-activated receptor gamma agonist. Arch Dermatol. 2009;145(12):1363–1366. doi: 10.1001/archdermatol.2009.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Callaghan A., Jr., Martinez G., Aragoneses H., Miranda-Romero A. Annular atrophic lichen planus. J Am Acad Dermatol. 2005;52(5):906–908. doi: 10.1016/j.jaad.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Nagao K., Chen K.R. A case of lupus erythematosus/lichen planus overlap syndrome. J Dermatol. 2006;33(3):187–190. doi: 10.1111/j.1346-8138.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- Nakamura R., Broce A.A., Palencia D.P., Ortiz N.I., Leverone A. Dermatoscopy of nail lichen planus. Int J Dermatol. 2013;52(6):684–687. doi: 10.1111/j.1365-4632.2011.05283.x. [DOI] [PubMed] [Google Scholar]
- Nanda A., Al-Ajmi H.S., Al-Sabah H., Al-Hasawi F., Alsaleh Q.A. Childhood lichen planus: a report of 23 cases. Pediatr Dermatol. 2001;18(1):1–4. doi: 10.1046/j.1525-1470.2001.018001001.x. [DOI] [PubMed] [Google Scholar]
- Neill S.M., Lewis F.M. Vulvovaginal lichen planus: a disease in need of a unified approach. Arch Dermatol. 2008;144(11):1502–1503. doi: 10.1001/archderm.144.11.1502. [DOI] [PubMed] [Google Scholar]
- Nnoruka E.N. Lichen planus in African children: a study of 13 patients. Pediatr Dermatol. 2007;24(5):495–498. doi: 10.1111/j.1525-1470.2007.00501.x. [DOI] [PubMed] [Google Scholar]
- Nousari H.C., Goyal S., Anhalt G.J. Successful treatment of resistant hypertrophic and bullous lichen planus with mycophenolate mofetil. Arch Dermatol. 1999;135(11):1420–1421. doi: 10.1001/archderm.135.11.1420. [DOI] [PubMed] [Google Scholar]
- Pai K., Pai S. Zosteriform lichen planus: case report of a rare variant of lichen planus. Our Dermatol Online. 2013;4(2):183. [Google Scholar]
- Pandhi D., Singal A., Bhattacharya S.N. Lichen planus in childhood: a series of 316 patients. Pediatr Dermatol. 2014;31(1):59–67. doi: 10.1111/pde.12155. [DOI] [PubMed] [Google Scholar]
- Perez-Rodriguez I.M., Garcia-Melendez M.E., Eichelmann K., Vazquez-Martinez O., Ocampo-Candiani J. Hyperpigmentation following treatment of frontal fibrosing alopecia. Case Rep Dermatol. 2013;5(3):357–362. doi: 10.1159/000357022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S., Mohan R., Ghanta S., Verma S., Agarwal N., Gupta N. Meteorological influences on the incidence of lichen planus in a north Indian population. J Oral Sci. 2013;55(4):311–318. doi: 10.2334/josnusd.55.311. [DOI] [PubMed] [Google Scholar]
- Rai R., Kaur I., Kumar B. Low-dose low-molecular-weight heparin in lichen planus. J Am Acad Dermatol. 2002;46(1):141–143. doi: 10.1067/mjd.2002.117389. [DOI] [PubMed] [Google Scholar]
- Rashid M.M., Sikder M.A., Hoque S., Kabir E. Linear lichen planus: A case report. J Pak Assoc Dermatol. 2008;18(4):241–244. [Google Scholar]
- Reich H.L., Nguyen J.T., James W.D. Annular lichen planus: a case series of 20 patients. J Am Acad Dermatol. 2004;50(4):595–599. doi: 10.1016/j.jaad.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Rieder E., Kaplan J., Kamino H., Sanchez M., Pomeranz M.K. Lichen planus pigmentosus. Dermatol Online J. 2013;19(12):20713. [PubMed] [Google Scholar]
- Salavastru C., Tiplica G.S. Therapeutic hotline: ulcerative lichen planus--treatment challenges. Dermatol Ther. 2010;23(2):203–205. doi: 10.1111/j.1529-8019.2010.01316.x. [DOI] [PubMed] [Google Scholar]
- Samrao A., Chew A.L., Price V. Frontal fibrosing alopecia: a clinical review of 36 patients. Br J Dermatol. 2010;163(6):1296–1300. doi: 10.1111/j.1365-2133.2010.09965.x. [DOI] [PubMed] [Google Scholar]
- Santos-Juanes J., Mas-Vidal A., Coto-Segura P., Sanchez del Rio J., Galache Osuna C. Pigmented actinic lichen planus successfully treated with intense pulsed light. Br J Dermatol. 2010;163(3):662–663. doi: 10.1111/j.1365-2133.2010.09857.x. [DOI] [PubMed] [Google Scholar]
- Sciallis G.F., Loprinzi C.L., Davis M.D. Progressive linear lichen planus and metastatic carcinoma. Br J Dermatol. 2005;152(2):399–401. doi: 10.1111/j.1365-2133.2005.06416.x. [DOI] [PubMed] [Google Scholar]
- Sekar C.S., Rai R., Karthika N., Laila A. Scle-lp overlap syndrome. Indian J Dermatol. 2011;56(2):209–210. doi: 10.4103/0019-5154.80420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J.K., Lee H.J., Lee D., Choi J.H., Sung H.S. A case of linear lichen planus pigmentosus. Ann Dermatol. 2010;22(3):323–325. doi: 10.5021/ad.2010.22.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrao V.V., Organ V., Pereira L., Vale E., Correia S. Annular lichen planus in association with crohn disease. Dermatol Online J. 2008;14(9):5–8. [PubMed] [Google Scholar]
- Sharma R., Maheshwari V. Childhood lichen planus: a report of fifty cases. Pediatr Dermatol. 1999;16(5):345–348. doi: 10.1046/j.1525-1470.1999.00074.x. [DOI] [PubMed] [Google Scholar]
- Sobel J.D. Treatment of vulvovaginal lichen planus with vaginal hydrocortisone suppositories. Curr Infect Dis Rep. 2002;4(6):507–508. doi: 10.1007/s11908-002-0036-9. [DOI] [PubMed] [Google Scholar]
- Tandon Y.K., Somani N., Cevasco N.C., Bergfeld W.F. A histologic review of 27 patients with lichen planopilaris. J Am Acad Dermatol. 2008;59(1):91–98. doi: 10.1016/j.jaad.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Tonsager M., Crutchfield C.E., III Atrophic lichen planus. Dermatol Nurs. 2004;16(1):73–74. [PubMed] [Google Scholar]
- Torres J.R., Torres J., Romero A., Reyes E., Hidalgo L.G. Lichen planus pigmentosus in patients with endocrinopathies and hepatitis C. J Am Acad Dermatol. 2013;68(4):AB139. [Google Scholar]
- Tosti A., Peluso A.M., Fanti P.A., Piraccini B.M. Nail lichen planus: clinical and pathologic study of twenty-four patients. J Am Acad Dermatol. 1993;28(5 Pt 1):724–730. doi: 10.1016/0190-9622(93)70100-8. [DOI] [PubMed] [Google Scholar]
- Vachiramon V., Suchonwanit P., Thadanipon K. Bilateral linear lichen planus pigmentosus associated with hepatitis C virus infection. Case Rep Dermatol. 2010;2(3):169–172. doi: 10.1159/000320775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G., Rose C., Sachse M.M. Clinical variants of lichen planus. J Dtsch Dermatol Ges. 2013;11(4):309–319. doi: 10.1111/ddg.12031. [DOI] [PubMed] [Google Scholar]
- Walton K.E., Bowers E.V., Drolet B.A., Holland K.E. Childhood lichen planus: demographics of a U.S. population. Pediatr Dermatol. 2010;27(1):34–38. doi: 10.1111/j.1525-1470.2009.01072.x. [DOI] [PubMed] [Google Scholar]
- Yamanaka Y., Akiyama M., Shibaki A., Kikuchi T., Shimizu H. Annular lichen planus: Study of the cellular mechanisms of annularity. Dermatology. 2004;208(4):335–338. doi: 10.1159/000077843. [DOI] [PubMed] [Google Scholar]
- Zhang R.Z., Zhu W.Y. Lichen planus pigmentosus over superficial leg veins. J Dtsch Dermatol Ges. 2011;9(7):540–541. doi: 10.1111/j.1610-0387.2011.07610.x. [DOI] [PubMed] [Google Scholar]


