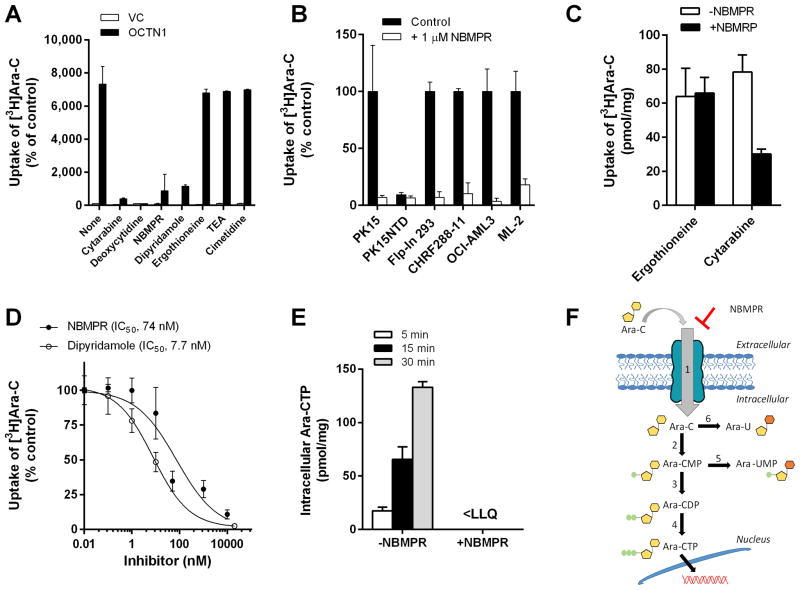
Fig. 3. Inhibition of OCTN1-mediated cytarabine transport.
Characterization of the transport of cytarabine (Ara-C; concentration, 1 μM; 5-min uptake) in the absence or presence of putative OCTN1 inhibitors (concentration, 10–50 μM) was performed in HEK293 cells transfected with an empty vector (VC) or OCTN1 (A). Influence of NBMPR on the transport of cytarabine in various cell lines (B) and primary human epidermal keratinocytes (C). Concentration-dependence of the inhibitory properties of NBMPR and dipyridamole on OCTN1-mediated transport of cytarabine was assessed over a 0.01–10 μM concentration range (5-min uptake) (D). The time-dependent influence of NBMPR (10 μM) on the formation of the pharmacologically-active metabolite cytarabine triphosphate (Ara-CTP) in cells exposed to cytarabine (10 μM; 5–30 min uptake) was determined by liquid chromatography-tandem mass spectrometry (E). Data are shown as mean values (bars or symbols) and SEM (error bars), using 6–15 observations per group. Solid lines represent a fit of the experimental data to an inverse non-linear maximum-effect model (panel D). Abbreviations: NBMPR, nitrobenzylmercaptopurine ribonucleoside; IC50, concentration required to inhibit OCTN1-mediated cytarabine transport by 50%; <LLQ, lower than the lower limit of quantitation of the analytical assay. A schematic of OCTN1-mediated transport, metabolism, and (in)activation of cytarabine in cells is shown in (F). 1, NBMPR-sensitive transport by OCTN1; 2, deoxycytidine kinase; 3, deoxycytidine monophosphate kinase; 4, nucleoside diphosphate kinase; 5, cytoplasmic 5′-nucleotidase; 6, cytidine deaminase.