Fig. 1.
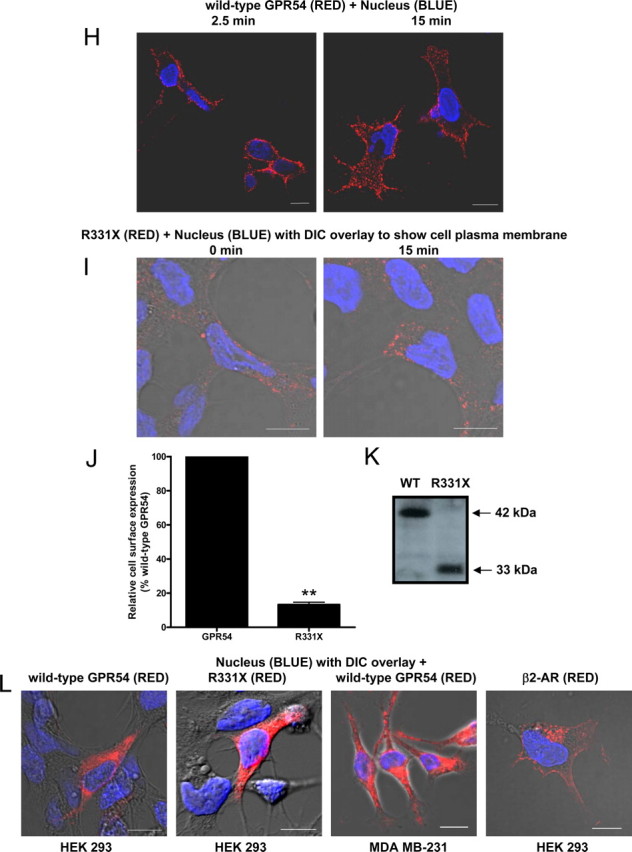
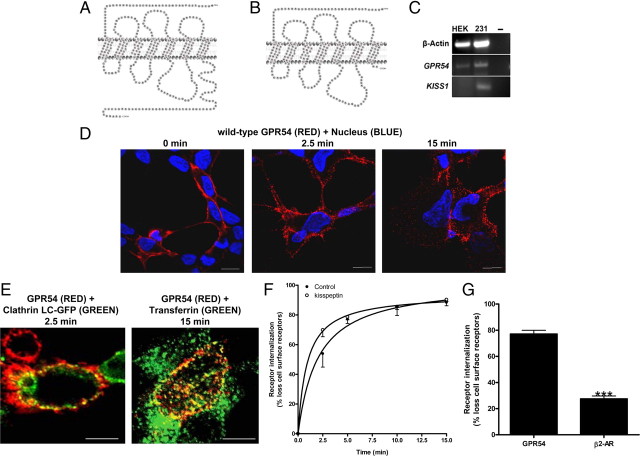
The expression, localization, and internalization of GPR54. Schematic of wild-type GPR54 (A) and R331X (B) showing absence of most of the Ct, except for two residues based on the predicted model. C, RT-PCR analysis of GPR54 and KISS1 expression in HEK 293 and MDA-MB-231 cells. Third lane shows water control. D, HEK 293 cells transiently expressing FLAG-GPR54 were surface labeled at 4 C (0 min) using rabbit anti-FLAG antibody followed by agonist treatment (100 nm Kp-10) at 37 C for 2.5 and 15 min. After fixation, surface GPR54 was detected by Alexa Fluor 568-conjugated antirabbit IgG in the nonpermeabilized cells. E, HEK 293 cells transiently transfected with 5 μg FLAG-GPR54 and 5 μg clathrin light chain-GFP (left panel) or 5 μg FLAG-GPR54 only (right panel) were labeled at 4 C using rabbit anti-FLAG primary antibody. Cells transfected with receptor only were also incubated with transferrin conjugate-488. After agonist treatment (100 nm Kp-10) at 37 C for 2.5 (left panel) and 15 mins (right panel), cells were fixed and surface GPR54 was detected by Alexa Fluor 568-conjugated antirabbit IgG. F, Time course for GPR54 basal or constitutive (closed circle) and Kp-10-stimulated (open circle) internalization. The data represent the mean ± se of nine independent experiments. HEK 293 cells transiently expressing GPR54 were surface labeled at 4 C using mouse anti-FLAG antibody. Cells were left untreated or incubated with 100 nm Kp-10 at 37 C for the indicated times and then fixed. Internalization was calculated as the percentage of loss of cell surface immunofluorescence over time and measured by flow cytometry. G, Bar chart showing receptor internalization of HEK 293 cells transiently transfected with FLAG-GPR54 or FLAG β2-AR after 5 min of treatment with 100 nm Kp-10 or 10 μm isoproterenol at 37 C. The data represent the mean ± se of three independent experiments. ***, P < 0.001 vs. GPR54. H, HEK 293 cells transiently expressing FLAG-GPR54 were surface labeled at 4 C (0 min) using rabbit anti-FLAG antibody followed by incubation in the absence of agonist at 37 C for 2.5 and 15 min. After fixation, surface GPR54 was detected by Alexa Fluor 568-conjugated antirabbit IgG in the nonpermeabilized cells. I, HEK 293 cells transiently expressing FLAG-R331X were surface labeled at 4 C (0 min) using rabbit anti-FLAG antibody followed by agonist treatment (100 nm Kp-10) at 37 C for 15 min. After fixation, surface receptor was detected by Alexa Fluor 568-conjugated antirabbit IgG in the nonpermeabilized cells. **, P < 0.01 vs. GPR54. J, Analysis of wild-type FLAG-GPR54 and R331X surface expression in nonstimulated cells. HEK 293 cells transiently expressing the receptors were surface labeled at 4 C using rabbit anti-FLAG antibody and fixed, and cell surface immunofluorescence was measured by flow cytometry. Data represent mean ± se for three independent experiments. K, Western blot analysis of wild-type (WT) FLAG-GPR54 and R331X. Protein was detected with the rabbit anti-FLAG primary antibody (L) HEK 293 cells transfected with FLAG-GPR54 (first panel), R331X (second panel), and FLAG β2-AR (fourth panel), and MDA-MB-231 cells transfected with FLAG-GPR54 (third panel) were fixed, permeabilized, and subjected to indirect immunofluorescent staining using rabbit anti-FLAG antibody followed by Alexa Fluor 568-conjugated antirabbit IgG. Scale bars, 10 μm. DIC, Differential interference contrast; WT, wild type.