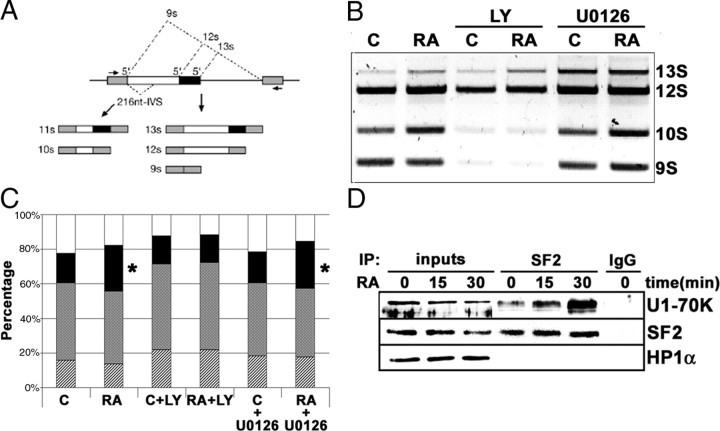
Fig. 7.
RA treatment influences the regulation of alternative mRNA splicing via activation of signaling pathways. A, Schematic representation of the alternative splicing forms in the E1A minigene reporter and the RT-PCR fragments obtained from the different mRNA isoforms generated. [Adapted from Ref. 40 ]. B, Effect of RA treatment on the regulation of splice site selection. After transfection of the E1A minigene reporter, neuroblastoma cells were treated with 1 μm RA (24 h), plus LY294002 (10 μm) or U0126 (10 μm) as indicated. Total RNA was obtained, and the different splicing variants were detected by RT-PCR. After electrophoresis in an agarose gel, the bands were stained with ethidium bromide. C, Control cells; RA, RA-treated cells; LY, cells treated with the PI3K inhibitor LY294002 (10 μm); U0126, cells treated with the MEK inhibitor U0126 (10 μm). C, Quantitative analysis of the experiment shown in Fig. 6B. The relative percentages of the different mRNA isoforms generated by alternative splicing and the effect of the different treatments are shown. The graph includes the data from three independent experiments. Stippled bars, 13S; shadowed bars; 12 S; solid bars, 10S; open bars, 9S. Asterisks on the 10S isoform in RA and RA+U0126 samples indicate statistically significant differences with respect to control (P = 0.002). All the other samples were not statistically different from control (ANOVA plus Bonferroni post hoc test). D, RA treatment enhanced the interaction between SF2/ASF and the U1 snRNP-70K protein. Nuclear extracts were prepared from neuroblastoma cells treated with 1 μm RA for 0, 15, and 30 min. About 200 μg of nuclear proteins was incubated with 4 μg of SF2/ASF monoclonal antibody (lanes labeled “SF2”). As control, a parallel reaction was set with 4 μg of an unrelated antibody (lane labeled “IgG”). Nuclear extracts (25 μg) from cells treated with 1 μm RA for the times indicated in the figure were included for comparison (lanes labeled “inputs”). Immunocomplexes were precipitated using TrueBlot antimouse IgG beads (eBioscience), following the manufacturer’s instructions, and suspended in sample buffer containing freshly added 50 mm DTT. After Western blot, the filter was developed with anti-U1 snRNP-70K protein (U1-70K), using horseradish peroxidase-conjugated rabbit TrueBlot secondary antibody (eBioscience). The filter was reprobed additionally with anti-SF2/ASF monoclonal antibody (SF2), using horseradish peroxidase-conjugated rabbit TrueBlot secondary antibody (eBioscience). As specificity control, the filter was reprobed with an unrelated anti-HP1α antibody (HP1 α). IP, Immunoprecipitation; LY, LY294002.