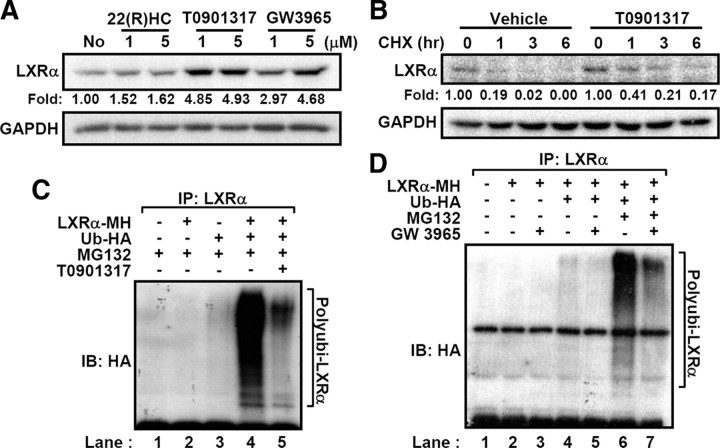
Fig. 2.
LXRα ubiquitination is decreased by LXR ligands. A, LXRα-expressing HEK293 cells were treated with LXR ligands such as 22(R)-HC, T0901317, and GW3965 for 3 h. Total lysates were subjected to Western blottings. Band intensities were calculated by the LabWorks software (UVP Bioimaging Systems), and fold induction of LXRα protein was normalized by glyceraldehyde-3-phosphate dehydrogenase protein level. B, T0901317 (5 μm) was pretreated to the LXRα-expressing HEK293 cells for 30 min and harvested at the indicated time periods after cycloheximide treatment. Protein level of LXRα was analyzed by Western blotting. C, Transfected HEK293 cells with LXRα and HA-tagged ubiquitin expression vectors were incubated with MG132 (20 μm) and/or T0901317 (5 μm) for 3 h. Total cell lysates were isolated and subjected to in vivo ubiquitination assays. D, Similarly, ubiquitination of LXRα was analyzed after GW3965 treatment (5 μm). All the experiments were independently repeated at least three times, and representative results were shown. CHX, Cycloheximide; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IB, immunoblotting; IP, immunoprecipitation.