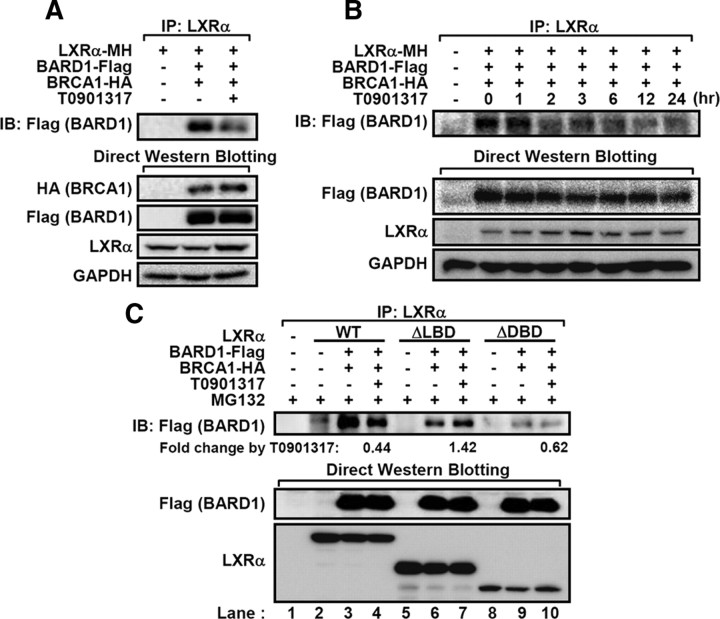
Fig. 4.
BARD1/BRCA1 interacts with LXRα in a ligand-dependent manner. A, HEK293 cells transfected with LXRα and BARD1/BRCA1 expression vectors were incubated with T0901317 (5 μm) and MG132 (20 μm) for 3 h, and total lysates were subjected to coimmunoprecipitation and Western blotting. B, Cotransfected HEK293 cells with LXRα and BARD1/BRCA1 were incubated with T0901317 (5 μm) and MG132 (20 μm) for the indicated periods. After harvesting, total cell lysates were coimmunoprecipitated with LXRα antibodies, and Western blotting was performed. C, The WT, ΔLBD, and ΔDBD LXRα were cotransfected with BARD1 and BRCA1. After treatment with T0901317 (5 μm) and MG132 (20 μm) for 3 h, coimmunoprecipitation assays were performed. Relative fold changes of immunoprecipitated BARD1 protein by T0901317 were indicated. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; IB, immunoblotting; IP, immunoprecipitation.