Abstract
Developmental and compartmentation studies were used to evaluate the relative roles of the oxidative pentose phosphate cycle, the Calvin cycle, and the glycolysis in cotyledons of radish (Raphanus sativus L.).
Glucose-6-P dehydrogenase, 6-P-gluconate dehydrogenase, glucose-6-P isomerase, and the NAD-dependent glyceraldehyde-3-P dehydrogenase were present in high activity in ungerminated seeds, increased about 2-fold during germination in the dark, and were slightly enhanced by light. In contrast, NADP-dependent glyceraldehyde-3-P dehydrogenase was developed to only a small degree in the dark, but increased severalfold in continuous white or far red light. The activity of phosphofructokinase was low throughout germination.
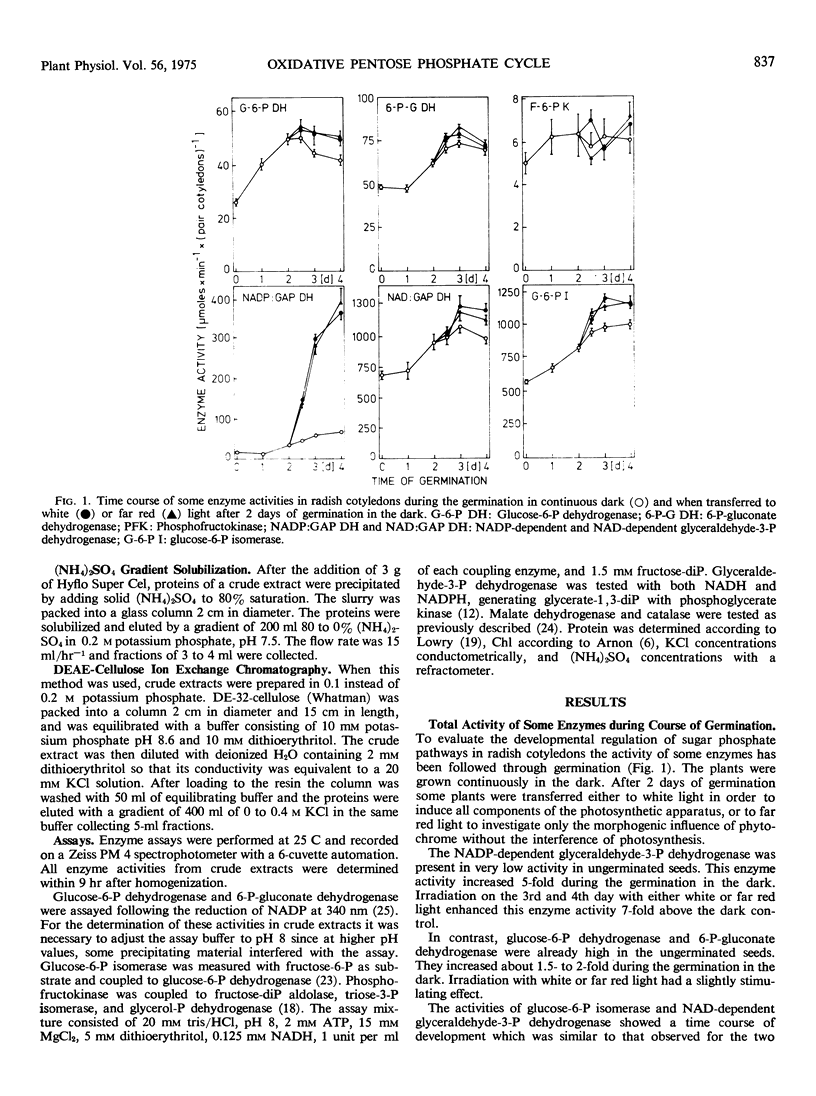
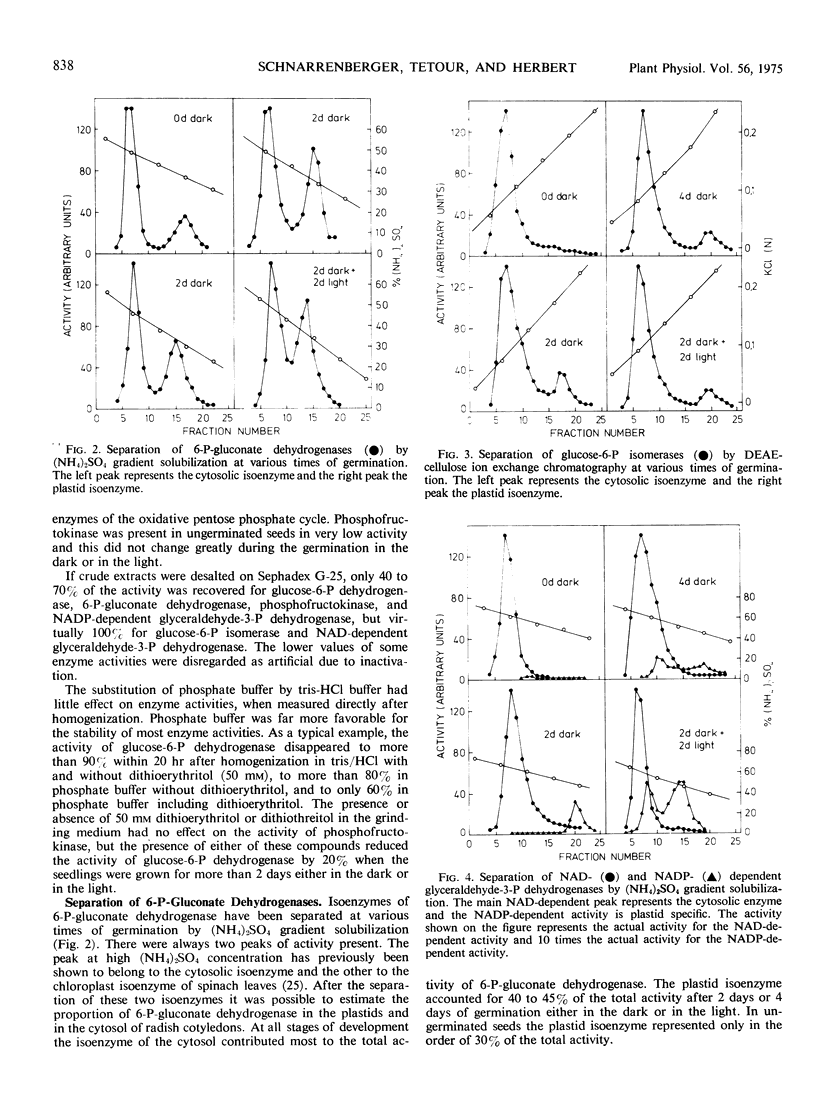
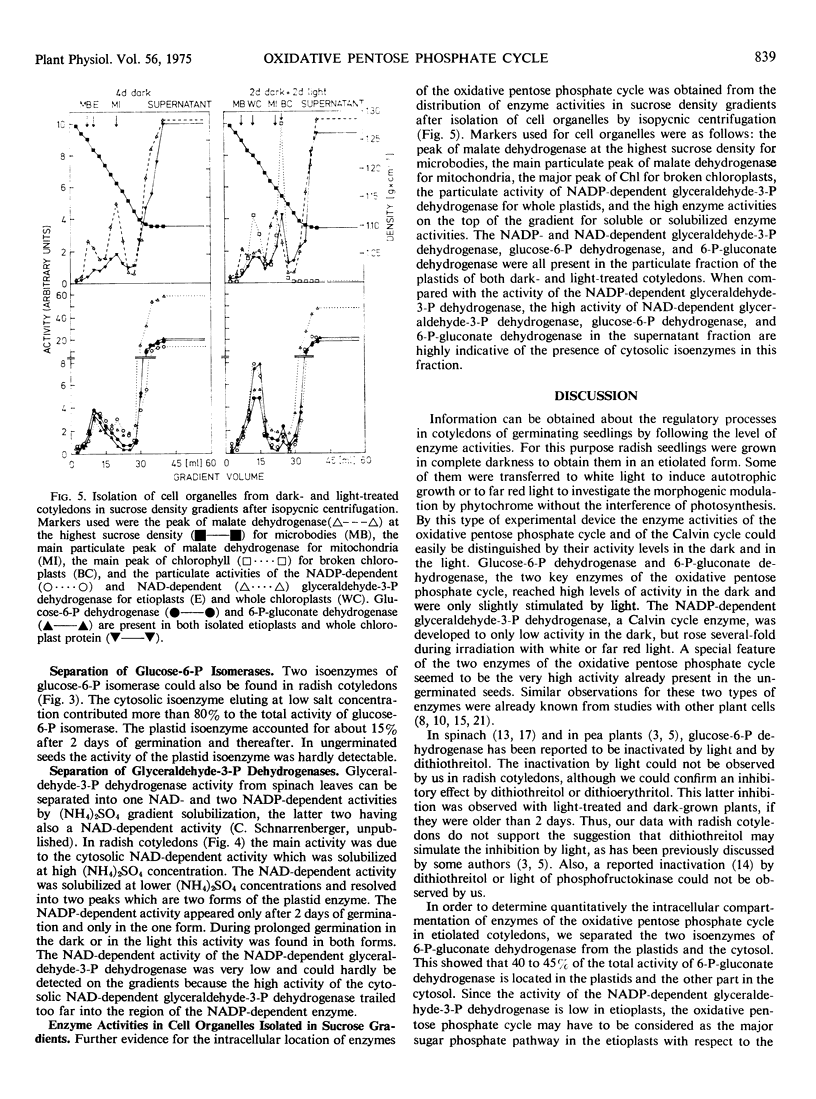
The separation of cell compartment-specific isoenzymes showed that, except in ungerminated seeds, the plastid enzyme accounted for 40 to 45% of the total activity of 6-P-gluconate dehydrogenase and for 15 to 20% of glucose-6-P isomerase. The remaining activity was due to the cytosolic isoenzymes. The presence of glucose-6-P dehydrogenase and 6-P-gluconate dehydrogenase in plastids was also established by their presence in the isolated organelle. The NAD-dependent glyceraldehyde-3-P dehydrogenase was mostly due to the cytosolic isoenzyme, whereas the NAD-dependent activity associated with the NADP-dependent glyceraldehyde-3-P dehydrogenase was very small.
The data indicate that the enzymes of the oxidative pentose phosphate cycle are present in the cytosol throughout germination. In the plastids these enzymes already became fully developed during early germination in the dark, whereas enzymes of the Calvin cycle increased only in the light. Glycolysis seemed to be of minor importance.
Full text
PDF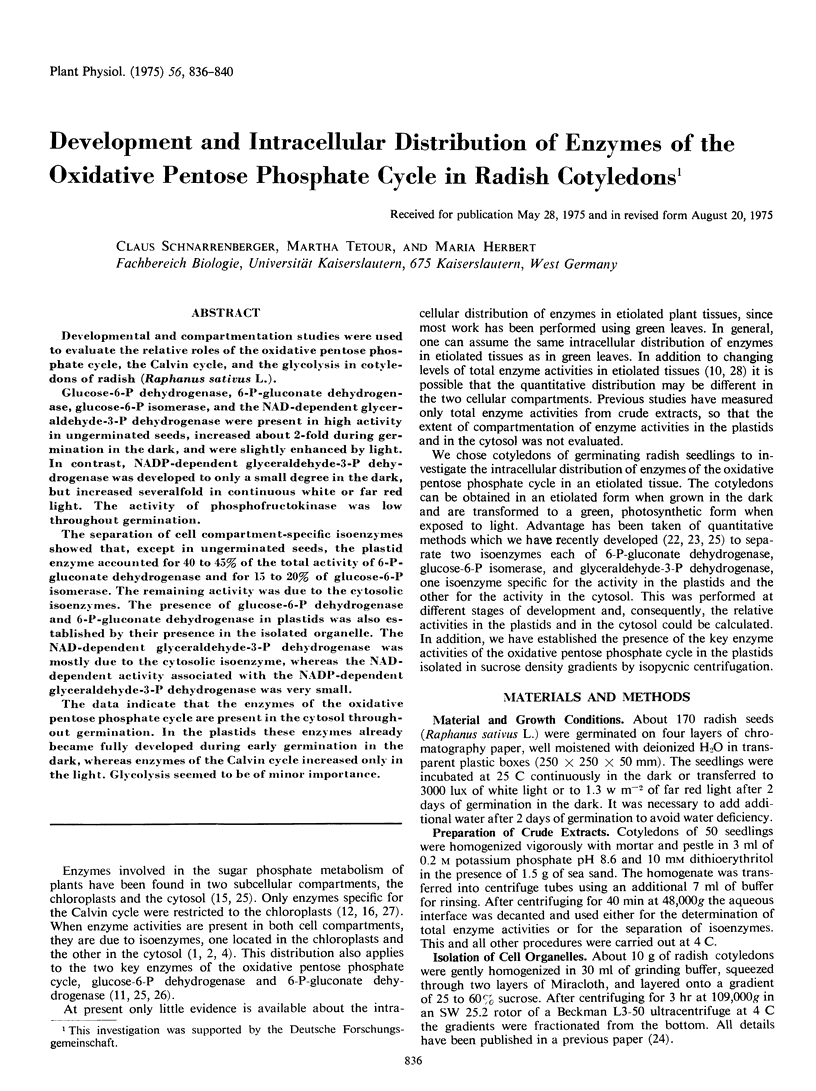

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., Advani V. R. Chloroplast and cytoplasmic enzymes: three distinct isoenzymes associated with the reductive pentose phosphate cycle. Plant Physiol. 1970 May;45(5):583–585. doi: 10.1104/pp.45.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E. Chloroplast and cytoplasmic enzymes. 3. Pea leaf ribose 5-phosphate isomerases. Biochim Biophys Acta. 1971 Apr 14;235(1):245–249. doi: 10.1016/0005-2744(71)90052-0. [DOI] [PubMed] [Google Scholar]
- Anderson L. E. Chloroplast and cytoplasmic enzymes. II. Pea leaf triose phosphate isomerases. Biochim Biophys Acta. 1971 Apr 14;235(1):237–244. doi: 10.1016/0005-2744(71)90051-9. [DOI] [PubMed] [Google Scholar]
- Anderson L. E., Ng T. C., Park K. E. Inactivation of pea leaf chloroplastic and cytoplasmic glucose 6-phosphate dehydrogenases by light and dithiothreitol. Plant Physiol. 1974 Jun;53(6):835–839. doi: 10.1104/pp.53.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalling M. J., Tolbert N. E., Hageman R. H. Intracellular location of nitrate reductase and nitrite reductase. II. Wheat roots. Biochim Biophys Acta. 1972 Dec 14;283(3):513–519. doi: 10.1016/0005-2728(72)90267-8. [DOI] [PubMed] [Google Scholar]
- FORTI G., TUA C., TOGNOLI L. Fractionation of oxidative particles of the pea stem. Biochim Biophys Acta. 1959 Nov;36:19–28. doi: 10.1016/0006-3002(59)90064-2. [DOI] [PubMed] [Google Scholar]
- Heber U., Hallier U. W., Hudson M. A. Untersuchungen zur intrazellulären Verteilungen von Enzymen und Substraten in der Blattzelle. II. Lokalisation von Enzymen des reduktiven und dem oxydativen Pentosephosphat-Zyklus in den Chloroplasten und Permeabilität der Chloroplasten-Membran gegenüber Metaboliten. Z Naturforsch B. 1967 Nov;22(11):1200–1215. [PubMed] [Google Scholar]
- Heber U., Pon N. G., Heber M. Localization of Carboxydismutase & Triosephosphate Dehydrogenases in Chloroplasts. Plant Physiol. 1963 May;38(3):355–360. doi: 10.1104/pp.38.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachru R. B., Anderson L. E. Inactivation of pea leaf phosphofructokinase by light and dithiothreitol. Plant Physiol. 1975 Feb;55(2):199–202. doi: 10.1104/pp.55.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Manabe K. A Rapid Phytochrome-dependent Reduction of Nicotinamide Adenine Dinucleotide Phosphate in Particle Fraction from Etiolated Bean Hypocotyl. Plant Physiol. 1973 May;51(5):982–983. doi: 10.1104/pp.51.5.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Development of Microbodies in Sunflower Cotyledons and Castor Bean Endosperm during Germination. Plant Physiol. 1971 Nov;48(5):566–574. doi: 10.1104/pp.48.5.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Two isoenzymes each of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in spinach leaves. Arch Biochem Biophys. 1973 Jan;154(1):438–448. doi: 10.1016/0003-9861(73)90077-5. [DOI] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A. Two isoenzymes of glucosephosphate isomerase from spinach leaves and their intracellular compartmentation. Eur J Biochem. 1974 Jun 1;45(1):77–82. doi: 10.1111/j.1432-1033.1974.tb03531.x. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Hatch M. D. Comparative studies on the activity of carboxylases and other enzymes in relation to the new pathway of photosynthetic carbon dioxide fixation in tropical grasses. Biochem J. 1967 Jun;103(3):660–665. doi: 10.1042/bj1030660. [DOI] [PMC free article] [PubMed] [Google Scholar]


