Abstract
Cell-nonautonomous effects of signaling in the nervous system of animals can influence diverse aspects of organismal physiology. We previously showed that phosphorylation of Ser49 of the α-subunit of eukaryotic translation initiation factor 2 (eIF2α) in two chemosensory neurons by PEK-1/PERK promotes entry of Caenorhabditis elegans into dauer diapause. Here, we identified and characterized the molecular determinants that confer sensitivity to effects of neuronal eIF2α phosphorylation on development and physiology of C. elegans. Isolation and characterization of mutations in eif-2Ba encoding the α-subunit of eIF2B support a conserved role, previously established by studies in yeast, for eIF2Bα in providing a binding site for phosphorylated eIF2α to inhibit the exchange factor eIF2B catalytic activity that is required for translation initiation. We also identified a mutation in eif-2c, encoding the γ-subunit of eIF2, which confers insensitivity to the effects of phosphorylated eIF2α while also altering the requirement for eIF2Bγ. In addition, we show that constitutive expression of eIF2α carrying a phosphomimetic S49D mutation in the ASI pair of sensory neurons confers dramatic effects on growth, metabolism, and reproduction in adult transgenic animals, phenocopying systemic responses to starvation. Furthermore, we show that constitutive expression of eIF2α carrying a phosphomimetic S49D mutation in the ASI neurons enhances dauer entry through bypassing the requirement for nutritionally deficient conditions. Our data suggest that the state of eIF2α phosphorylation in the ASI sensory neuron pair may modulate internal nutrient sensing and signaling pathways, with corresponding organismal effects on development and metabolism.
Keywords: Caenorhabditis elegans, dauer, eIF2α, phosphorylation, sensory neurons, translational control
PHOSPHORYLATION of the α-subunit of eukaryotic translation initiation factor 2 (eIF2α) is an evolutionarily conserved mechanism of translation control in eukaryotic cells that is pivotal for regulation of gene expression during stress (reviewed in Sonenberg and Hinnebusch 2009). In Saccharomyces cerevisiae, eIF2α phosphorylation by the eIF2α kinase GCN2 promotes cellular adaptation to nutrient deficiency by attenuating global protein synthesis and preferentially upregulating translation of transcripts that are associated with stress alleviation (Hinnebusch 2005). In mammals, four eIF2α kinases have been identified and are activated by endogenous and environmental cues that include amino acid starvation (GCN2), endoplasmic reticulum (ER) protein-folding imbalance (PERK), presence of foreign double-stranded RNA (PKR), and heme deprivation (HRI); hence constituting a homeostatic mechanism termed the integrated stress response (reviewed in Sonenberg and Hinnebusch 2009).
Phosphorylated eIF2α [eIF2(αP)] attenuates protein synthesis by sequestering the guanine nucleotide exchange factor eukaryotic translation initiation factor 2B (eIF2B), the activity of which is required for the GTP-binding eIF2 to initiate translation (reviewed in Sonenberg and Hinnebusch 2009). While the cellular consequences of eIF2α phosphorylation have been thoroughly delineated at the biochemical level (reviewed in Wek et al. 2006; Sonenberg and Hinnebusch 2009), recent studies have also demonstrated tissue-specific roles of phosphorylation of eIF2α in animal physiology and disease. For instance, eIF2α phosphorylation and the eIF2α kinase GCN2 have been shown to regulate intestinal homeostasis and suppress gut inflammation (Cao et al. 2014; Ravindran et al. 2016). Recent studies have also highlighted complex physiological roles of eIF2α phosphorylation in the mammalian central nervous system. Specifically, essential amino acid deprivation induces phosphorylation of eIF2α via GCN2 in the mammalian anterior piriform cortex to promote aversion to an amino acid-deficient diet (Hao et al. 2005; Maurin et al. 2005). Neuronal eIF2α phosphorylation also governs synaptic plasticity and learning by modulating expression of proteins involved in both long-term potentiation and depression at hippocampal synapses (Costa-Mattioli et al. 2007; Di Prisco et al. 2014). In addition, human genetic analyses have revealed that mutations in genes encoding translation initiation components regulating eIF2 activity, such as subunits of the exchange factor eIF2B and the γ-subunit of eIF2, are associated with defects in myelination in the brain and mental disability, respectively (reviewed in Bugiani et al. 2010; Borck et al. 2012). Collectively, these findings indicate that, in addition to maintaining cellular homeostasis, neuronal eIF2α phosphorylation may exert cell-nonautonomous effects on organismal physiology and disease.
In Caenorhabditis elegans, environmental stressors, including those that induce translation attenuation such as nutrient limitation, trigger a state of developmental arrest termed dauer diapause, involving profound adaptations in metabolism, reproduction, and behavior (Cassada and Russell 1975). The genetic study of the dauer-developmental decision of C. elegans has served as an experimental paradigm for understanding how environmental cues influence organismal physiology through conserved neuroendocrine signaling pathways, including insulin and transforming growth factor-β (TGFβ) (reviewed in Hu 2007; Fielenbach and Antebi 2008). In a previous study, we characterized the mechanism by which the daf-28(sa191) mutation, previously isolated and molecularly characterized as a mutation in a gene encoding an insulin ligand (Malone and Thomas 1994; Li et al. 2003), causes constitutive dauer entry. The R37C substitution in the DAF-28 insulin peptide causes ER stress specifically in the ASI chemosensory neurons, activating the unfolded protein response (UPR) regulator PEK-1/PERK, which phosphorylates a conserved regulatory Ser49 in eIF2α in the ASI chemosensory neuron pair to promote entry into dauer diapause (Kulalert and Kim 2013). Because DAF-28 itself has been previously established to function redundantly to inhibit dauer formation (Cornils et al. 2011; Hung et al. 2014), UPR activation and PEK-1-mediated eIF2α phosphorylation act in conjunction with the lack of functional antidauer DAF-28 in the daf-28(sa191) mutant to confer a strong, constitutive dauer-entry phenotype in this mutant background.
Here, to further characterize the mechanism underlying the organismal response to this neuron-specific eIF2α phosphorylation, we sought to isolate mutations that could suppress the constitutive dauer-entry phenotype of the daf-28(sa191) mutant. We have identified conserved mechanisms mediating sensitivity to eIF2α phosphorylation in the nervous system of C. elegans. We also characterize developmental and metabolic phenotypes of animals expressing an eIF2α transgene carrying a phosphomimetic S49D mutation, which suggest a cell-nonautonomous role for the state of eIF2α phosphorylation in the ASI sensory neuron pair in modulating the organismal response to nutrient deficiency.
Materials and Methods
C. elegans strains
C. elegans strains were maintained as previously described (Brenner 1974). Strains used in this study are listed in Supplemental Material, Table S3 in File S1.
Suppressor mutant isolation and characterization
Mutagenesis using ethyl methanesulfonate was performed on the starting strain carrying the daf-28(sa191) allele. F2 generation eggs from 16 independent F1-generation pools were plated onto NGM plates seeded with Escherichia coli strain OP50. The plates were screened for F2 animals that, unlike the starting strain, failed to enter dauer diapause 48 hr later at 25°. Specifically, we isolated L4 or young adult F2 animals. The recovered mutants were retested and selected based on viability and the ability to give rise to a largely nondauer population in subsequent generations.
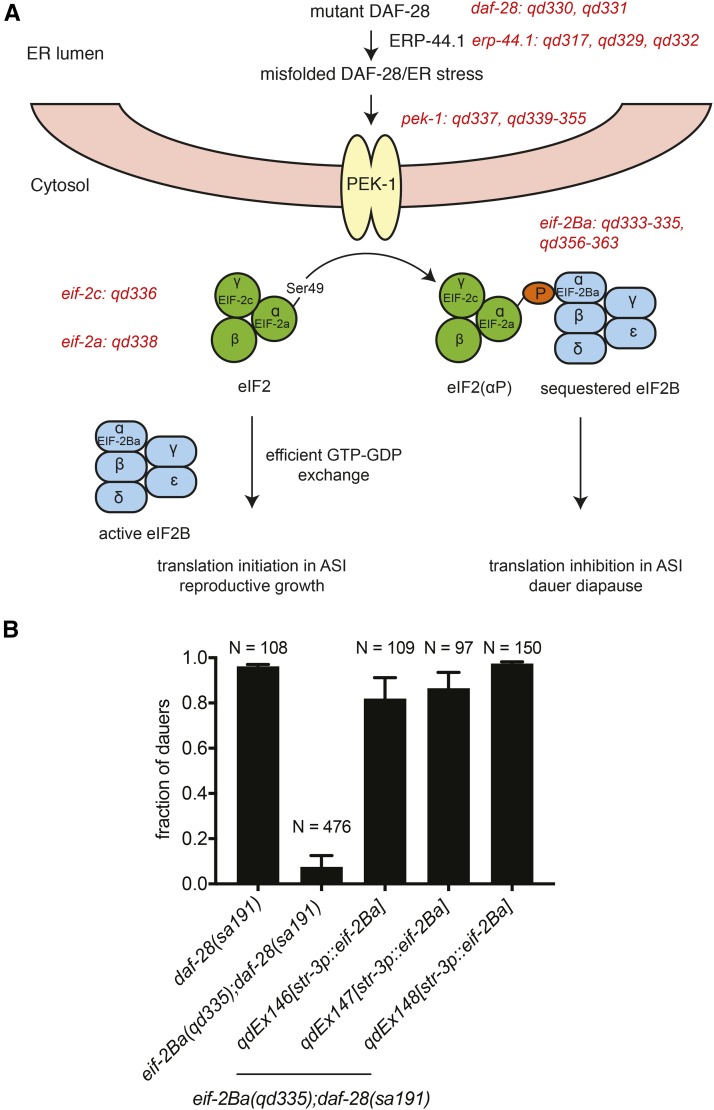
We then crossed the mutants into the starting strain to determine mode of inheritance. For recessive suppressor mutants, complementation testing was performed using the previously characterized suppressor daf-28(sa191);pek-1(ok275) strain (Kulalert and Kim 2013) and the eif-2Ba(qd335);daf-28(sa191) mutant (see recessive eif-2Ba alleles in Table S2 in File S1). Representative dominant mutants were outcrossed at least three times to the starting strain and were submitted for whole genome resequencing and bioinformatics analyses, performed by BGI Americas. We then identified nucleotide polymorphisms that were unique to each mutant, and used existing deletion or presumptive null alleles [daf-28(tm2308), erp-44.1(gk411949), and eif-2Ba(pk720)] to determine whether loss-of-function mutations in the genes contribute to the phenotypic suppression. Sanger sequencing was used to identify additional alleles of the candidate genes in the remaining mutants. For eif-2Ba, we also performed cell-specific rescue to confirm that EIF-2Ba functions in the ASI neurons to promote dauer formation in response to neuronal eIF2α phosphorylation (Figure 1B).
Figure 1.
Identification of genes functioning in the ASI neurons to promote the dauer developmental decision in response to neuronal UPR activation and subsequent eIF2α phosphorylation. (A) Schematic illustrating the alleles and gene products characterized in the study. Molecular identities of the alleles are listed in Table 1. (B) Fractions of the animals with indicated genotypes that form dauers at 25°. eif-2Ba was overexpressed specifically in the ASI neurons using the ASI-specific promoter str-3p. eif-2Ba(qd335);daf-28(sa191) presented were pooled from nontransgenic animals, all of which exhibited similar dauer-entry frequencies. Plotted is mean ± SD. N represents total number of animals for each of the three independent transgenic lines.
Constructs and generation of transgenic animals
The promoter of the gpa-4 gene was used as an ASI-specific promoter to express transgenes (Jansen et al. 1999; Bishop and Guarente 2007). Importantly, the gpa-4 promoter was able to drive heterologous gene expression to mediate organismal phenotypes in both larval and adult stages, specifically dauer formation and diet-restriction-mediated lifespan extension (Bishop and Guarente 2007; Kulalert and Kim 2013).
We also used str-3p to drive neuron-specific expression of eif-2Ba in Figure 1B. Plasmids containing str-3p::eif-2Ba::unc-54 3′UTR (70 ng/μl) and ofm-1p::GFP (80 ng/μl) were used to generate transgenic animals, and three independent lines were recovered and propagated.
As described in Evans (2006), gamma radiation was employed to integrate the qdIs10[gpa-4p::eIF2α(S49D)] extrachromosomal arrays. The animals carrying the integrated arrays were then outcrossed nine times. Animals carrying extrachromosomal (Kulalert and Kim 2013; Figure S5 in File S1) or integrated arrays both exhibited similar starvation-like phenotypes.
Dauer formation assay
Six to eight gravid animals were picked to individual well-seeded, 6-cm NGM plates, allowed to lay eggs at the assay temperature for 3–6 hr, and removed. Live E. coli strain OP50 was used as a food source. For the assays conducted at 25°, dauer and L4 larvae were scored at 48 hr after the egg-lay midpoint, as at this time point all the animals have passed the predauer stages and, for the dauer-constitutive mutants that exit dauer, the dauer larvae have not resumed reproductive development. Dauers were discriminated from nondauers based on radial shrinkage of the body, the absence of pharyngeal pumping, and an overall dark appearance (Cassada and Russell 1975). To minimize variation in environmental conditions that could influence dauer formation, the same position in the incubator was used in all the experiments for temperature consistency, and the population density on each plate was controlled by the number of gravid adults laying eggs and the duration of egg laying.
Unless otherwise noted, all dauer formation assays were done without ascarosides. In Figure 5, B and C, 100 μl of the pheromone mix consisting of synthetic ascarosides #2, #3, #5, and #8 (Pungaliya et al. 2009) was added onto 3.5-cm plates made with Noble agar and without peptone. Concentrated heat-killed or live E. coli OP50 were then seeded onto the plates. Egg laying was done at 25° using around three adults of indicated genotypes for 3–5 hr, and scoring took place 72 hr afterward. The average total number of animals per plate was ∼40–60.
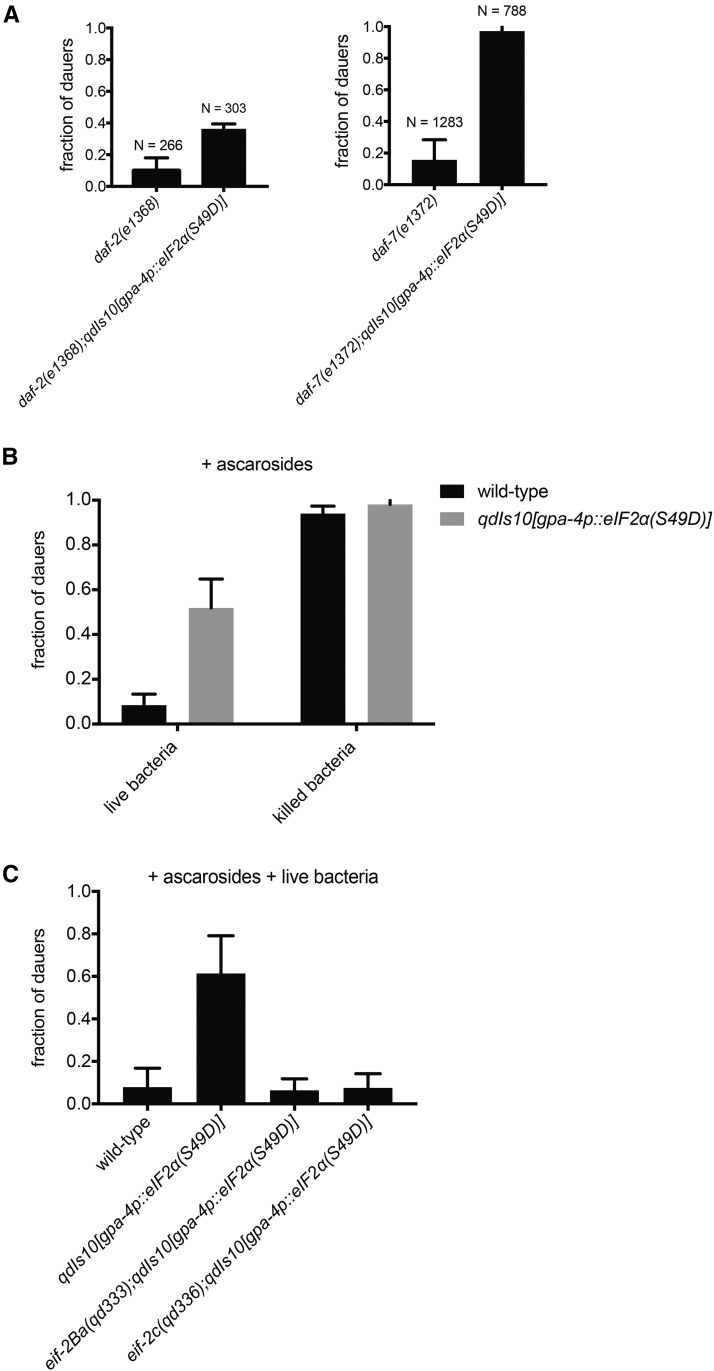
Figure 5.
Aberrant eIF2α phosphorylation in the ASI chemosensory neurons modulates the dauer developmental decision in response to neuroendocrine and environmental signals. (A) Fractions of the indicated neuroendocrine pathway mutants, in the presence or absence of the qdIs10[gpa-4p::eIF2α(S49D)] transgene, that form dauer at 25°. N represents total number of animals for each genotype. Results shown here are representative of three independent experiments. (B) Fractions of the animals from wild-type vs. qdIs10[gpa-4p::eIF2α(S49D)] backgrounds that form dauer at 25°, in the presence of ascarosides and indicated food sources. Results shown here are representative of three independent experiments. (C) Fractions of the animals from wild-type vs. qdIs10[gpa-4p::eIF2α(S49D)] backgrounds that form dauer at 25°, in the presence of ascarosides and live bacteria. Results shown here are representative of five independent experiments. (A–C) Plotted is mean ± SD.
Brood size assay
Individual L4 animals were placed onto NGM plates seeded with E. coli strain OP50 at 25°. Each animal was then transferred to individual plates on a daily basis for at least 5 days. The number of eggs laid or progeny hatched was then scored every day. The results shown were based on multiple animals from at least two independent experiments.
RNA interference-dependent assays
RNA interference (RNAi) by bacterial feeding using E. coli HT115 bacteria was carried out as reported. All vectors used in this study were validated by Sanger sequencing. For each experiment, bacteria expressing the empty L4440 vector (negative control RNAi), the L4440-derived unc-22 RNAi vector (positive control RNAi, based on the twitching phenotype induced by unc-22 knockdown), and the respective L4440-derived translation regulatory factor gene RNAi vectors were included. L4 animals were fed on RNAi bacteria plates for 72 hr at 16°, and the F1-generation animals were scored for fractions that reached L4 and subsequent fertile adulthood at 25°. Results shown were based on multiple independent experiments.
Microscopy
Animals at indicated developmental stages were mounted with 10 mM sodium azide onto slides with a 2% agarose pad. The slides were viewed using an AxioImager Z1 fluorescence microscope (Carl Zeiss, Thornwoord, NY) with indicated objectives. The fluorescence signals were recorded by a charge-coupled-device camera (AxioCam), using constant exposure time without saturation for each experiment.
For lipid storage visualization, Sudan Black staining was performed using a protocol adapted from Kimura et al. (1997). Specifically, animals were fixed in 1% formaldehyde, stained with 50% Sudan Black solution, and washed in M9 buffer four times before mounting.
Data availability
Strains are available upon request. The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results and Discussion
Isolation and characterization of mutations that suppress the dauer-constitutive phenotype of daf-28(sa191)
We mutagenized ∼40,000 genomes of daf-28(sa191) animals and isolated mutants that exhibited suppression of the dauer-constitutive phenotype of the starting strain (Figure S1 in File S1). We then employed complementation testing and whole-genome sequencing to identify candidate genes. Our prior study suggested that expression of the mutant R37C DAF-28 insulin peptide in the ASI neurons causes ER stress, activating the UPR and leading to activation of PERK/PEK-1, which phosphorylates eIF2α (Kulalert and Kim 2013). Consistent with our model, we isolated mutations affecting each of these steps (Figure 1A).
Mutations likely affecting the phosphorylation of eIF2α induced by the expression of toxic mutant R37C DAF-28:
First, we isolated second-site, revertant mutations in the gene encoding the mutant DAF-28 insulin (Figure 1A and Table 1). We identified a presumptive null allele of daf-28, which carries an additional missense mutation in the start codon (M1X R37C), preventing translation initiation of the toxic peptide. The absence of the toxic mutant DAF-28 likely abrogates the ER protein-folding imbalance that triggers neuronal eIF2α phosphorylation and dauer formation through PEK-1. Consistent with this hypothesis, we note that the analysis of transheterozygote animals carrying two distinct alleles of daf-28 suggests that this allele of daf-28 behaves similarly to the null daf-28(tm2308) deletion allele (Figure S2 in File S1). We also isolated another allele of daf-28, which results in a premature truncation of the peptide (R37C Q81X). Transheterozygote analysis suggests that this allele acts similarly to the wild-type daf-28 allele (Figure S2 in File S1); leading us to speculate that the 16-aa truncation, while having no effect on DAF-28 function, may restore pairings of cysteine residues, circumventing the ER-toxic consequences of exposed reactive thiol moieties of unpaired cysteines that promote dauer formation. The recovery of the loss-of-function and revertant alleles of daf-28 further underscores insulin misprocessing as a source of ER homeostasis perturbation, and suggests proteotoxic contributions of unpaired cysteine, as in the case of the Akita diabetes mouse model (Figure S2 in File S1; Wang et al. 1999).
Table 1. Isolation of mutations that suppress the constitutive dauer-entry phenotype induced by phosphorylation of eIF2α in two sensory neurons in the daf-28(sa191) mutant.
| Gene | Mammalian ortholog | Number of recovered alleles | Suppressor genotype | Molecular alteration | Fraction of dauers (mean ± SD) | Total number of animals |
|---|---|---|---|---|---|---|
| daf-28 | β-type insulin | 2 | daf-28(sa191)a | N/A | 0.99 ± 0.02 | 465 |
| daf-28(sa191qd330) | DAF-28(M1I R37C) | 0 | 228 | |||
| daf-28(sa191qd331) | DAF-28(R37C Q81X) | 0 | 214 | |||
| erp-44.1 (c30h7.2) | ERp44 | 3 | erp-44.1(qd317);daf-28(sa191) | Splice site mutationb | 0.005 ± 0.012 | 433 |
| erp-44.1(qd329);daf-28(sa191) | ERP-44.1(W4X) | 0.026 ± 0.031 | 347 | |||
| pek-1 | PERK | 18 | daf-28(sa191);pek-1(qd337) | PEK-1(R649X) | 0 | 231 |
| daf-28(sa191);pek-1(qd342) | PEK-1(R519X) | 0 | 210 | |||
| eif-2a (y37e3.10) | eIF2α | 1 | eif-2a(qd338);daf-28(sa191) | EIF-2a(S49F) | 0 | 131 |
| eif-2Ba (zk1098.4) | eIF2Bα | 11 | eif-2Ba(qd333);daf-28(sa191) | EIF-2Ba(E28K) | 0.002 ± 0.003 | 580 |
| eif-2Ba(qd334);daf-28(sa191) | EIF-2Ba(T41I) | 0.01 ± 0.01 | 472 | |||
| eif-2Ba(qd335);daf-28(sa191) | EIF-2Ba(M1a)b | 0.06 ± 0.01 | 318 | |||
| eif-2c (y39g10ar.8) | eIF2γ | 1 | eif-2c(qd336);daf-28(sa191) | EIF-2c(S443L) | 0 | 119 |
Starting strain.
See further descriptions of molecular identity in Table S1 in File S1.
Second, we isolated three presumptive null alleles of a previously uncharacterized gene c30h7.2 (Figure 1A and Table 1). The suppression of the dauer-entry phenotype was also confirmed by a previously generated null allele of c30h7.2, gk411949 [fraction of c30h7.2(gk411949);daf-28(sa191) dauers = 0.02 ± 0.02; N = 228]. The gene encodes an ortholog of mammalian ERp44, which is a member of the protein-disulfide isomerase family (Anelli et al. 2002, 2003). ERp44 is involved in quality control of several ER client proteins (Higo et al. 2005; Freyaldenhoven et al. 2012; Hisatsune et al. 2015; Yang et al. 2016). Importantly, ERp44 has been shown to directly interact with proinsulin in mouse insulinoma cells (Pottekat et al. 2013). Because loss-of-function mutations of c30h7.2 suppressed the organismal consequences of ER toxicity, we hypothesized that ERp44 may participate in formation or retention of the toxic DAF-28(R37C)-derived complexes that activate the UPR to promote dauer entry. Consistent with this hypothesis, we observed reduction of neuronal UPR activation in the absence of ERp44, suggesting that the isomerase contributes to the ER toxicity triggered by the mutant neuronal insulin (Figure S3 in File S1). Our genetic analysis of the C. elegans ERp44 points to a conserved function of the disulfide isomerase in modulating maturation of insulin (Figure S3 in File S1; Pottekat et al. 2013).
Third, as anticipated by our prior study (Kulalert and Kim 2013), we isolated 18 alleles of pek-1, which encodes the C. elegans ortholog of mammalian eIF2α kinase PERK that is activated by the toxic-peptide-mediated disruption of protein-folding homeostasis in the ER (Figure 1A and Table 1). In addition to nonsense substitutions that lead to premature truncation of PEK-1, the remaining of the recovered pek-1 alleles harbor alterations in the residues in the conserved cytoplasmic kinase and ER luminal domains of PEK-1 (Figure S1 and Table S1 in File S1).
Fourth, we recovered an allele of eIF2α (eif-2a/y37e3.10) that results in an S49F substitution at the conserved Ser49 target of eIF2α kinases (Figure 1A and Table 1). Suppression of the dauer-entry phenotype by this eIF2α allele further corroborates our genetic analysis of phosphomimetic (S49D) and unphosphorylatable (S49A) transgenes expressed in the ASI neuron pair, which implicated a pivotal role for eIF2α in promoting entry into dauer diapause (Kulalert and Kim 2013).
Mutations in eIF2Bα/EIF-2Ba altering sensitivity to eIF2α phosphorylation:
Translation initiation requires guanine nucleotide exchange on eIF2 mediated by the multimeric eIF2B (reviewed in Sonenberg and Hinnebusch 2009). Molecular genetic studies in yeast have established that eIF2Bα is a nonessential, regulatory subunit that provides a binding site for eIF2(αP) to inhibit the eIF2B guanine exchange factor (GEF) activity required for translation initiation (Yang and Hinnebusch 1996).
We isolated 11 distinct suppressor mutations in the gene encoding the C. elegans ortholog of the α-subunit of eIF2B (eif-2Ba), zk1098.4 (Table 1). Two presumptive null alleles of eif-2Ba also suppressed the constitutive dauer-entry phenotype [see qd335 in Table 1; for the eif-2Ba(pk720) deletion allele, fraction of eif-2Ba(pk720);daf-28(sa191) dauers = 0.02 ± 0.02; N = 133], consistent with the dispensable, regulatory role of GCN3/eIF2Bα in S. cerevisiae (Yang and Hinnebusch 1996). Overexpression of eif-2Ba specifically in the ASI neurons was able to restore the constitutive dauer-entry phenotype of the eif-2Ba(qd335);daf-28(sa191) mutant, indicating that EIF-2Ba functions in the two neurons to promote the dauer developmental decision in response to neuronal eIF2α phosphorylation (Figure 1B). Most of the eif-2Ba alleles isolated conferred the suppression in a dominant fashion, similar to the eif-2Ba deletion allele (Table S2 in File S1). The dominant nature of the eif-2Ba deletion allele in suppressing the organismal response to eIF2α phosphorylation suggests that eIF2B susceptibility to eIF2(αP) is readily perturbed by reduced eIF2Bα dosage. The eif-2Ba mutations that confer the dominant-suppressor phenotype similar to the loss-of-function allele likely affect residues that are critical for eIF2Bα functional and structural integrity (Table S2 in File S1). Additionally, because eIF2Bα forms a homodimer as part of the eIF2B holoenzyme, the altered eIF2Bα may exert a dominant-negative effect that prevents proper dimerization or holoenzyme formation (Kashiwagi et al. 2016).
We note that four eif-2Ba mutations alter residues that are conserved not only among eIF2Bα across species but also among the equivalent domains in the other regulatory subunits of eIF2B that recognize eIF2(αP), β and δ, suggesting pivotal regulatory functions (Table S2 in File S1). A number of previously characterized eIF2(αP)-insensitive mutations in yeast eIF2B α-, β- and, δ-subunits also affect residues and domains shared among all three regulatory subunits (Pavitt et al. 1997). Of note, a number of S. cerevisiae hypomorphic alleles of GCD7/eIF2Bβ and GCD2/eIF2Bδ that render the yeast cells refractory to the inhibitory effects of eIF2α phosphorylation have been characterized (Vazquez de Aldana et al. 1993). While we isolated 11 loss-of-function alleles of eif-2Ba, no alleles of the genes encoding the other two regulatory subunits, β and δ, were recovered. We observed that the β- and δ-subunits of eIF2B, unlike the α-subunit, are essential for C. elegans development (Figure 2C). Our data suggest a conserved molecular target and mechanism in response to eIF2α phosphorylation in C. elegans (Figure 1A).
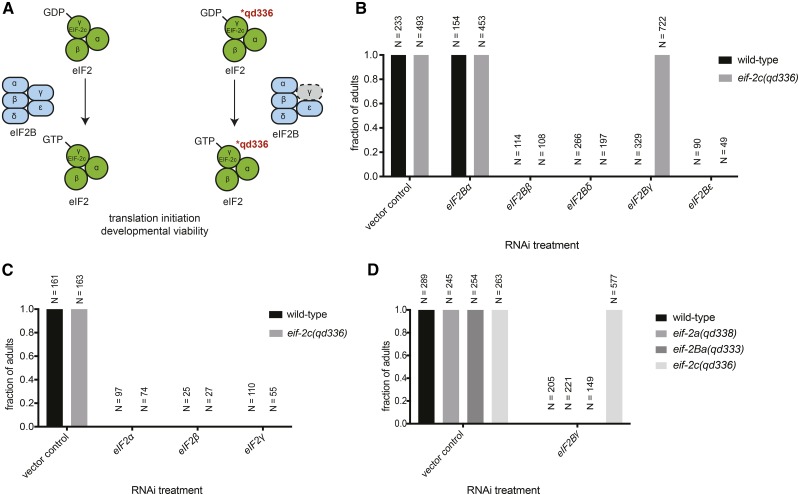
Figure 2.
The eif-2c(qd336) allele, encoding a mutant C. elegans ortholog of the γ-subunit of eIF2, alters sensitivity to eIF2α phosphorylation and translational requirements of eIF2B. (A) Schematic illustrating the qd336 variant of EIF-2c and its alteration of function in the context of translation initiation. (B) Fractions of the animals from wild-type vs. eif-2c(qd336) backgrounds that reach reproductive maturation under RNAi conditions that downregulate indicated eIF2B subunits, representative of three independent experiments. eIF2Bα is the only eIF2B subunit that is dispensable for viability. (C) Fractions of the animals from wild-type vs. eif-2c(qd336) backgrounds that reach reproductive maturation under RNAi conditions that downregulate indicated eIF2 subunits. (D) Fractions of the animals with indicated genotypes conferring eIF2(αP) insensitivity that reach reproductive maturation under RNAi conditions that downregulate eIF2Bγ. (B–D) Plotted is mean ± SD. N represents total number of animals for each condition.
Remarkably, >120 mutations in genes encoding human eIF2B subunits have been associated with vanishing white matter disease, which involves hypomyelination in the central nervous system (reviewed in Bugiani et al. 2010). The enrichment of neuropathology-associated eIF2B mutant alleles suggests critical roles of eIF2B subunits in regulating homeostasis and myelin formation function of glial cells in the mammalian brain (Lin et al. 2014). We also note that the eIF2B regulatory δ-subunit has been identified as the molecular target of the drug ISRIB that renders mammalian neurons refractory to eIF2α phosphorylation to enhance memory formation (Sekine et al. 2015; Sidrauski et al. 2015). These findings illustrate critical roles of eIF2B-mediated translational control in the nervous system of higher animals, in addition to the worm model.
Isolation and characterization of an unusual mutation in eIF2γ/EIF-2c:
The translation factor eIF2 is a G-protein complex comprised of three distinct subunits, α, β, and γ (reviewed in Sonenberg and Hinnebusch 2009). We identified a mutation in the C. elegans ortholog of the guanine nucleotide-binding γ-subunit of eIF2 (eif-2c), y39g10ar.8 (Figure 1A and Table 1), which conferred dauer suppression in a dominant manner, suggesting alteration of function of this essential factor. The qd336 mutation alters a conserved serine residue in domain III of eIF2γ (Roll-Mecak et al. 2004), resulting in an S443L substitution.
The isolation of an unusual allele of eif-2c(qd336) that can confer insensitivity to the effects of eIF2(αP) was reminiscent of a previously characterized mutation in GCD11(K250R), the yeast ortholog of eIF2γ, which obviates the need for the GEF eIF2B in protein synthesis (Erickson and Hannig 1996; Erickson et al. 2001). Such diminished reliance on eIF2B would relieve translation initiation from regulation by eIF2(αP)-mediated eIF2B sequestration. We examined if the eif-2c(qd336) mutation could also enable eIF2B-independent translation initiation by performing RNAi knockdown of individual eIF2B subunits to assess whether the eIF2γ mutant can bypass the requirement for eIF2B during larval development. RNAi knockdown of expression of the essential subunits eIF2Bβ, eIF2Bδ, eIF2Bγ and eIF2Bε inhibited larval development in the wild-type background (Figure 2B). Strikingly, we observed that the eif-2c(qd336) mutant developed normally and reached reproductive maturation in the absence of eIF2Bγ, while still failing to survive when expression of the other essential eIF2B subunits had been impaired by RNAi (Figure 2, A and B).
We note that the ability to circumvent the requirement for eIF2Bγ is unique to the eif-2c(qd336) mutant, and does not occur in the other eIF2(αP)-resistant mutants isolated from the screen (Figure 2D). The S443L substitution in eIF2γ also confers insensitivity to eIF2α phosphorylation via a distinct mechanism from the aforementioned S. cerevisiae GCD11(K250R) mutation (Erickson and Hannig 1996; Erickson et al. 2001), as the C. elegans eIF2γ(S443L) mutation did not result in growth impairment or bypass of the essential functions of eIF2α and all essential eIF2B subunits (Figure 2, B and C, compared to Erickson et al. 2001). Indeed, the eif-2c(qd336) mutant still requires the key catalytic subunit eIF2Bε which mediates the nucleotide-exchange activity (Figure 2B). Because EIF-2c(S443L) can functionally substitute for the eIF2Bγ subunit, but not for eIF2Bε (Figure 2B), the alternative mechanism underlying eIF2(αP) resistance is likely to involve a molecular event catalyzed exclusively or predominantly by eIF2Bγ, which is not the GTP-GDP exchange that is largely mediated by eIF2Bε (Pavitt et al. 1998). The eIF2Bγ subunit has been shown to also participate in the displacement of the translational regulatory factor eIF5 that prevents GDP dissociation from the translation-incompetent eIF2-GDP complex, thus antagonizing the exchange activity of eIF2B required for translation initiation (Jennings et al. 2016). It is plausible that the S443L change in eIF2γ facilitates the displacement of eIF5, bypassing the requirement for eIF2Bγ in dissociating eIF5 from eIF2 to promote translation-competent eIF2 complex formation. The augmented dissociation of eIF5 contributed by the mutant eIF2γ(S443L) may diminish the requirement for this specific eIF2B function in eIF5 displacement, altering sensitivity to reduced eIF2B activity when eIF2α is phosphorylated. Furthermore, in addition to sequestering eIF2B, phosphorylation of eIF2α has been postulated to enhance the abundance of the translation-incompetent eIF2-eIF5 complex (Jennings and Pavitt 2010). Therefore, the eIF2γ(S443L) alteration that destabilizes and depletes the eIF2(αP)-induced, translation-incompetent eIF2-eIF5 complexes would also undermine such translation inhibitory consequences of eIF2α phosphorylation.
We have demonstrated that the eIF2Bγ-specific functional or structural roles are dispensable in the eif-2c(qd336) mutant background (Figure 2B), likely resulting in reduced dependence on eIF2B and thus diminishing the inhibitory effects of eIF2(αP) on eIF2B functions and translation initiation (Figure 2A). We note that our mechanistic interpretations and distinctions among different eIF2 subunit mutants are mainly derived from the extensive genetic and biochemical studies conducted in yeast, in light of the high evolutionary conservation of residues and domains across eukaryotes. Importantly, our genetic characterization of the mechanistically distinct eif-2c(qd336) mutant unambiguously demonstrates that a subset of eIF2B’s catalytic functions is dispensable for translation initiation and organismal viability, enabling eIF2B-compensatory mechanisms to bypass the translational regulation by eIF2α phosphorylation that targets eIF2B.
Physiological consequences of phosphomimetic eIF2α(S49D) expression in the ASI neurons
Having defined mutants unable to respond to the effects of eIF2α phosphorylation, we sought to next examine the effects of increased eIF2α phosphorylation. Based on the insights gained from the study of the daf-28(sa191) mutant in which eIF2α phosphorylation is induced in the ASI neuron pair, we sought to further investigate the effects of increased eIF2α phosphorylation specifically in the ASI neuron pair. We examined animals carrying an integrated transgene expressing phosphomimetic C. elegans eIF2α(S49D) in the ASI neuron pair, which resulted in readily observed effects on growth, reproduction, metabolism, and the dauer developmental decision.
Expression of phosphomimetic eIF2α(S49D) in the ASI neuron pair mimics organismal phenotypes associated with nutrient deficiency:
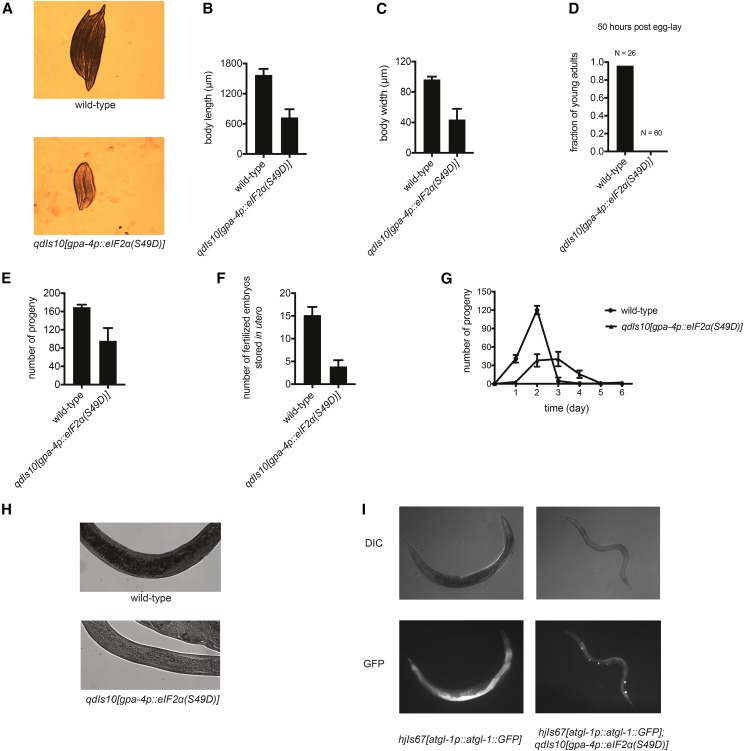
In adult hermaphrodite animals, expression of phosphomimetic eIF2α in the ASI neuron pair resulted in small body size (Figure 3, A–C), consistent with diminished growth rate (Figure 3D). The animals expressing eIF2α(S49D) in the ASI neurons also exhibited clear appearances, consistent with the observed diminished fat storage (Figure 3H). We also noted a significantly reduced brood size and an extension of the egg-laying period in the animals carrying the neuron-specific phosphomimetic eIF2α transgene, in comparison to the wild-type control (Figure 3, E and G). Unlike wild-type animals that stored 10–15 fertilized eggs in utero, the animals with ectopic neuronal eIF2α phosphorylation harbored a drastically reduced number of fertilized embryos (Figure 3F). The impairment in development and progeny production was independent of rearing temperature. Notably, these reproductive and metabolic defects are reminiscent of those occurring in animals that were defective in feeding or subjected to unfavorable conditions that promote reallocation of resources and germ cell death, such as starvation (Avery 1993; Angelo and Van Gilst 2009). Further corroborating the cell-nonautonomous influence of neuronal eIF2α phosphorylation on gene expression and metabolism, we observed downregulation of atgl-1, which encodes a lipase that functions in the intestine (Figure 3I). Such alteration in gene expression in distal tissues may suggest an adaptive role of the organismal consequences of neuron-specific eIF2(αP), for instance in preventing exhaustion of the energy reserve.
Figure 3.
Constitutive eIF2α phosphorylation in ASI impairs growth, metabolism, and reproduction; phenocopying the organismal responses to starvation. (A) Bright-field imaging of age-matched adult animals from wild-type vs. qdIs10[gpa-4p::eIF2α(S49D)] backgrounds. (B) Body length measurements, based on 10 representative, age-matched animals from wild-type vs. qdIs10[gpa-4p::eIF2α(S49D)] backgrounds. Plotted is mean ± SD. (C) Body width measurements, based on 10 representative, age-matched animals from wild-type vs. qdIs10[gpa-4p::eIF2α(S49D)] backgrounds. Plotted is mean ± SD. (D) Growth rate of animals with indicated genotypes at 25°. Young adults refer to animals that have undergone the fourth larval molt. Notably, fertilized embryos start to become visible in utero in these animals. (E) Total number of progeny production in the animals from wild-type vs. qdIs10[gpa-4p::eIF2α(S49D)] backgrounds, representative of three independent experiments. Plotted is mean ± SD. (F) Number of fertilized embryos observed in the hermaphrodite animals from wild-type vs. qdIs10[gpa-4p::eIF2α(S49D)] backgrounds. Plotted is mean ± SD. (G) Egg-laying period of the animals from wild-type vs. qdIs10[gpa-4p::eIF2α(S49D)] backgrounds, representative of three independent experiments. Plotted is mean ± SD. (H) Lipid staining of age-matched adult animals with indicated genotypes. (I) Fluorescence microscopy of the animals with indicated genotypes carrying the hjIs67[atgl-1p::atgl-1::GFP] transgene. The cotransformation marker of qdIs10[gpa-4p::eIF2α(S49D)], ofm-1p::GFP, also expresses GFP in the coelomocytes.
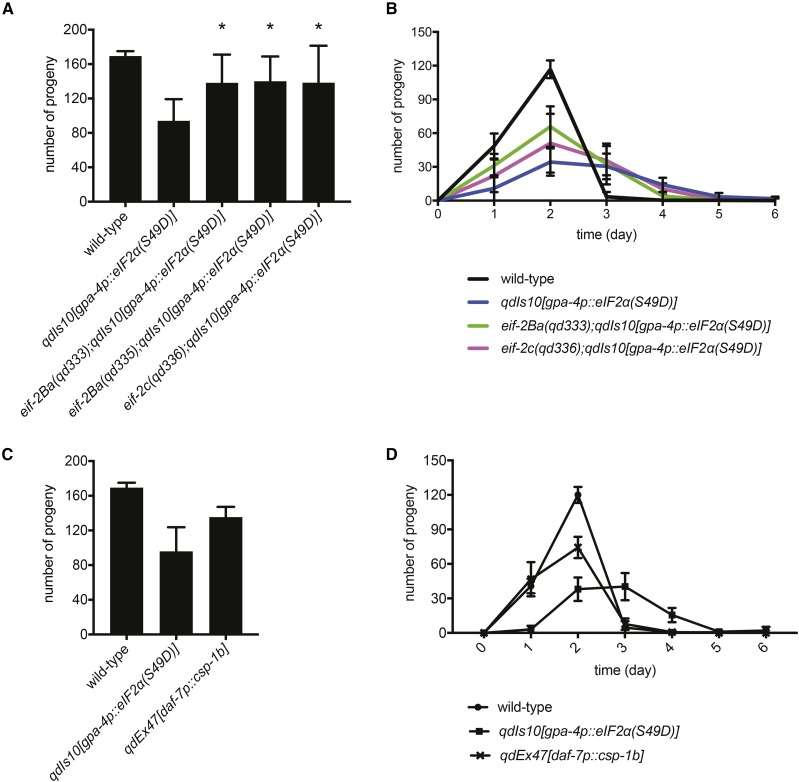
Expression of wild-type and unphosphorylatable versions of eIF2α in the ASI neuron pair did not result in diminished progeny production or an extended egg-laying period, indicating that the reproductive impairment is specific to phosphorylation status (Figure S5 in File S1). In addition, the systemic defects driven by the neuronal phosphomimetic eIF2α were significantly suppressed by the eif-2Ba and eif-2c mutations that confer insensitivity to eIF2α phosphorylation (Figure 4, A and B). The suppression of diverse physiological effects of ectopic neuronal eIF2(αP) by eif-2Ba and eif-2c mutations (Figure 1, Figure 4, A and B, and Figure 5C) underscores the roles of the C. elegans eIF2Bα and eIF2γ in mediating organismal sensitivity to phosphorylation of eIF2α throughout life. Moreover, we observed that genetic ablation of the ASI neurons, unlike constitutive eIF2α phosphorylation, had no effect on nondauer growth and reproduction (Figure 4, C and D); suggesting that general neuronal dysfunction or death did not account for the systemic phenotypes in response to the phosphorylation state of eIF2α.
Figure 4.
The effects of constitutive eIF2α phosphorylation in ASI on organismal physiology are distinct from those conferred by ASI ablation and are mediated by molecular determinants of sensitivity to eIF2α phosphorylation. (A) Total number of progeny production of the qdIs10[gpa-4p::eIF2α(S49D)] animals carrying indicated mutations that confer eIF2(αP) insensitivity. * P < 0.01, as determined by two-tailed Student’s t-test [qdIs10 vs. qdIs10 in the presence of eIF2(αP)-insensitive mutations]. (B) Egg-laying period of the animals with indicated genotypes. (C) Total number of progeny production of the animals, the ASI neurons of which have been genetically ablated. csp-1b, encoding a caspase ortholog, was expressed under the ASI-specific promoter daf-7p in the qdEx47 transgene. Ablation of ASI was confirmed by dauer entry (Bargmann and Horvitz 1991). Only animals that had entered dauer diapause and recovered were assayed for progeny production. (D) Egg-laying period of the animals with indicated genotypes. (A–D) Plotted is mean ± SD.
Expression of phosphomimetic eIF2α in the ASI neuron pair acts with pheromone to promote dauer entry even under optimal bacterial food conditions:
We also observed that the animals carrying the qdIs10 transgene expressing phosphomimetic eIF2α in the ASI neuron pair, while not exhibiting the constitutive-entry phenotype at the standard assay population density, were more prone to entering dauers when the plates became crowded without food deprivation. Additionally, constitutive neuronal eIF2α phosphorylation was sufficient to enhance dauer formation in the insulin- and TGFβ-deficient genetic backgrounds, which partially sensitize the animals to form dauer larvae (Figure 5A). Overexpression of wild-type eIF2α, unlike the phosphomimetic variant, has been shown to have no effect on the dauer decision (Kulalert and Kim 2013); indicating that the developmental effect elicited by the qdIs10 transgene is specific to phosphorylation status. Taken together, these observations suggest that, during larval development, ASI-specific eIF2α phosphorylation synergizes with other previously characterized dauer-promoting inputs, including crowding and aberrant neuroendocrine signaling levels, to trigger entry into diapause.
A robust method to induce dauer formation in wild-type animals in the laboratory is treatment with assortments of ascarosides in the presence of heat-killed E. coli, as live bacteria confer inhibitory effects on dauer formation (Figure 5B; Jeong et al. 2005; Kim et al. 2009; McGrath et al. 2011; Park et al. 2012). The molecular basis of the requirement for heat-killed bacteria to efficiently induce dauer entry in the presence of dauer pheromone in the laboratory remains unclear. Nondauer animals grown on heat-killed E. coli exhibit impaired growth and reproduction, indicative of exposure to a poor nutritional source. It is thus plausible that the nutritionally deficient nature of heat-killed bacteria contributes to the robust dauer formation observed in the pheromone assay. Because constitutive eIF2α phosphorylation in the ASI neurons, which integrate both dauer pheromone and food signals, phenocopies responses to food scarcity in adult animals (Figure 3); we hypothesize that ASI-specific phosphorylation of eIF2α may also confer similar antigrowth effects during larval development, mimicking nutritionally deficient conditions that enhance dauer formation in the presence of dauer pheromone.
Corroborating our hypothesis, the animals carrying the ASI-specific phosphomimetic eIF2α(S49D) transgene formed dauers efficiently even in the presence of dauer pheromone and live bacteria, the progrowth food source that largely suppressed dauer entry in wild-type control (Figure 5C). Importantly, constitutive phosphorylation of eIF2α in ASI is not sufficient to promote dauer formation without the presence of dauer pheromone, indicating that aberrant neuronal eIF2α-phosphorylation status may only specifically modulate food-dependent sensitivity to the ascarosides. The ability of the qdIs10 transgene to promote dauer formation in response to dauer pheromone, despite the presence of the progrowth live E. coli, is dependent on the organismal sensitivity to neuronal eIF2α phosphorylation mediated by eIF2Bα and eIF2γ, as the corresponding eIF2(αP)-insensitive mutations suppressed the prodauer effects (Figure 5C). These observations highlight the roles of the molecular determinants of eIF2α phosphorylation, EIF-2Ba and EIF-2c, in mediating the dauer developmental decision not only in the context of neuronal UPR activation (Figure 1A and Table 1), but also in the modulation of food-dependent sensitivity to ascarosides by constitutive neuronal eIF2α phosphorylation (Figure 5C). Collectively, our findings indicate that aberrant eIF2α status in the sensory neurons can confer dramatic responses that phenocopy starvation in adults, as well as modulating or synergistic effects on the dauer developmental decision.
Phosphorylation state of eIF2α in the ASI sensory neuron pair regulates organismal physiology
eIF2α phosphorylation is an evolutionarily conserved mechanism of nutrient detection in the unicellular yeast, as uncharged transfer RNAs symptomatic of amino acid deprivation activate the GCN2 eIF2α kinase. (Hinnebusch 1997; Wek et al. 2006). While cellular consequences of eIF2α phosphorylation have been extensively characterized in yeast (reviewed in Sonenberg and Hinnebusch 2009), physiological systemic roles of the conserved translational control mechanism have been highlighted in recent studies, particularly in the nervous system. Specifically, a broad array of complex biological processes in mammals including foraging, learning, addiction, and imprinting have been shown to be modulated by eIF2α phosphorylation in the central nervous system (Hao et al. 2005; Costa-Mattioli et al. 2007; Stern et al. 2013; Batista et al. 2016; Huang et al. 2016). We have demonstrated that constitutive phosphorylation of eIF2α in the ASI chemosensory neurons results in defects in growth rate, progeny production, and fat storage (Figure 4). These systemic impairments are similar to those manifested when the animals are exposed to nutritionally limited conditions, reminiscent of the phenotypes exhibited by the eat mutants that are defective in food intake (Avery 1993; Lakowski and Hekimi 1998; Figure S4 in File S1). That the organismal effects of neuronal eIF2(αP) on the adult worms mimic a starvation response is consistent with the triggering molecular event (eIF2α phosphorylation) and its site of action (ASI neurons). Our data therefore suggest that stress-induced eIF2α phosphorylation in the nervous system plays a critical role in intertissue communication and coordination, regulating diverse physiological outputs in C. elegans. Such systemic effects of eIF2α-mediated translational control illustrate how multicellular organisms may coopt conserved stress signaling pathways that maintain cellular homeostasis to modulate organism-wide responses to environmental fluctuations and challenges.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.117.200568/-/DC1.
Acknowledgments
We thank Joshua Meisel and Zoë Hilbert for strains carrying the qdEx47 transgene. We thank the Caenorhabditis Genetics Center, which is supported by the National Institutes of Health–National Center for Research Resources, and S. Mitani and the National BioResource Project for providing strains used in this study. This work was supported by National Institutes of Health grant GM-084477 (to D.H.K.).
Author contributions: W.K. and D.H.K. conceived research. W.K. performed experiments. H.S. assisted with experiments in Figure 2 and Figure 3. Y.K.Z. and F.C.S. provided ascarosides used in Figure 5. W.K. and D.H.K. wrote the manuscript.
Footnotes
Communicating editor: P. Sengupta
Literature Cited
- Anelli T., Alessio M., Bachi A., Bergamelli L., Bertoli G., et al. , 2003. Thiol-mediated protein retention in the endoplasmic reticulum: the role of ERp44. EMBO J. 22: 5015–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anelli T., Alessio M., Mezghrani A., Simmen T., Talamo F., et al. , 2002. ERp44, a novel endoplasmic reticulum folding assistant of the thioredoxin family. EMBO J. 21: 835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelo G., Van Gilst M. R., 2009. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science 326: 954–958. [DOI] [PubMed] [Google Scholar]
- Avery L., 1993. The genetics of feeding in Caenorhabditis elegans. Genetics 133: 897–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann C. I., Horvitz H. R., 1991. Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science 251: 1243–1246. [DOI] [PubMed] [Google Scholar]
- Batista G., Johnson J. L., Dominguez E., Costa-Mattioli M., Pena J. L., 2016. Translational control of auditory imprinting and structural plasticity by eIF2α. Elife 5: e17197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop N. A., Guarente L., 2007. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature 447: 545–549. [DOI] [PubMed] [Google Scholar]
- Borck G., Shin B. S., Stiller B., Mimouni-Bloch A., Thiele H., et al. , 2012. eIF2γ mutation that disrupts eIF2 complex integrity links intellectual disability to impaired translation initiation. Mol. Cell 48: 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugiani M., Boor I., Powers J. M., Scheper G. C., van der Knaap M. S., 2010. Leukoencephalopathy with vanishing white matter: a review. J. Neuropathol. Exp. Neurol. 69: 987–996. [DOI] [PubMed] [Google Scholar]
- Cao S. S., Wang M., Harrington J. C., Chuang B. M., Eckmann L., et al. , 2014. Phosphorylation of eIF2α is dispensable for differentiation but required at a posttranscriptional level for paneth cell function and intestinal homeostasis in mice. Inflamm. Bowel Dis. 20: 712–722. [DOI] [PubMed] [Google Scholar]
- Cassada R. C., Russell R. L., 1975. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 46: 326–342. [DOI] [PubMed] [Google Scholar]
- Cornils A., Gloeck M., Chen Z., Zhang Y., Alcedo J., 2011. Specific insulin-like peptides encode sensory information to regulate distinct developmental processes. Development 138: 1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M., Gobert D., Stern E., Gamache K., Colina R., et al. , 2007. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell 129: 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Prisco G. V., Huang W., Buffington S. A., Hsu C. C., Bonnen P. E., et al. , 2014. Translational control of mGluR-dependent long-term depression and object-place learning by eIF2α. Nat. Neurosci. 17: 1073–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson F. L., Hannig E. M., 1996. Ligand interactions with eukaryotic translation initiation factor 2: role of the gamma-subunit. EMBO J. 15: 6311–6320. [PMC free article] [PubMed] [Google Scholar]
- Erickson F. L., Nika J., Rippel S., Hannig E. M., 2001. Minimum requirements for the function of eukaryotic translation initiation factor 2. Genetics 158: 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, T. C., 2006 Transformation and injection (April 6, 2006), WormBook, ed. The C. elegans Research Community WormBook, /10.1895/wormbook.1.108.1, http://www.wormbook.org.
- Fielenbach N., Antebi A., 2008. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 22: 2149–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyaldenhoven S., Li Y., Kocabas A. M., Ziu E., Ucer S., et al. , 2012. The role of ERp44 in maturation of serotonin transporter protein. J. Biol. Chem. 287: 17801–17811. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hao S., Sharp J. W., Ross-Inta C. M., McDaniel B. J., Anthony T. G., et al. , 2005. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science 307: 1776–1778. [DOI] [PubMed] [Google Scholar]
- Higo T., Hattori M., Nakamura T., Natsume T., Michikawa T., et al. , 2005. Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell 120: 85–98. [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G., 1997. Translational regulation of yeast GCN4. A window on factors that control initiator-trna binding to the ribosome. J. Biol. Chem. 272: 21661–21664. [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G., 2005. Translational regulation of GCN4 and the general amino acid control of yeast. Annu. Rev. Microbiol. 59: 407–450. [DOI] [PubMed] [Google Scholar]
- Hisatsune C., Ebisui E., Usui M., Ogawa N., Suzuki A., et al. , 2015. ERp44 exerts redox-dependent control of blood pressure at the ER. Mol. Cell 58: 1015–1027. [DOI] [PubMed] [Google Scholar]
- Hu, P. J., 2007 Dauer (August 8, 2007), WormBook, ed. The C. elegans Research Community WormBook, /10.1895/wormbook.1.144.1, http://www.wormbook.org.
- Huang W., Placzek A. N., Viana Di Prisco G., Khatiwada S., Sidrauski C., et al. , 2016. Translational control by eIF2α phosphorylation regulates vulnerability to the synaptic and behavioral effects of cocaine. eLife 5: e12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung W. L., Wang Y., Chitturi J., Zhen M., 2014. A Caenorhabditis elegans developmental decision requires insulin signaling-mediated neuron-intestine communication. Development 141: 1767–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen G., Thijssen K. L., Werner P., van der Horst M., Hazendonk E., et al. , 1999. The complete family of genes encoding G proteins of Caenorhabditis elegans. Nat. Genet. 21: 414–419. [DOI] [PubMed] [Google Scholar]
- Jennings M. D., Pavitt G. D., 2010. eIF5 has GDI activity necessary for translational control by eIF2 phosphorylation. Nature 465: 378–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings M. D., Kershaw C. J., White C., Hoyle D., Richardson J. P., et al. , 2016. eIF2β is critical for eIF5-mediated GDP-dissociation inhibitor activity and translational control. Nucleic Acids Res. 44: 9698–9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong P. Y., Jung M., Yim Y. H., Kim H., Park M., et al. , 2005. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature 433: 541–545. [DOI] [PubMed] [Google Scholar]
- Kashiwagi K., Takahashi M., Nishimoto M., Hiyama T. B., Higo T., et al. , 2016. Crystal structure of eukaryotic translation initiation factor 2B. Nature 531: 122–125. [DOI] [PubMed] [Google Scholar]
- Kim K., Sato K., Shibuya M., Zeiger D. M., Butcher R. A., et al. , 2009. Two chemoreceptors mediate developmental effects of dauer pheromone in C. elegans. Science 326: 994–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K. D., Tissenbaum H. A., Liu Y., Ruvkun G., 1997. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277: 942–946. [DOI] [PubMed] [Google Scholar]
- Kulalert W., Kim D. H., 2013. The unfolded protein response in a pair of sensory neurons promotes entry of C. elegans into dauer diapause. Curr. Biol. 23: 2540–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowski B., Hekimi S., 1998. The genetics of caloric restriction in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 95: 13091–13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Kennedy S. G., Ruvkun G., 2003. daf-28 encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF-2 signaling pathway. Genes Dev. 17: 844–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Pang X., Huang G., Jamison S., Fang J., et al. , 2014. Impaired eukaryotic translation initiation factor 2B activity specifically in oligodendrocytes reproduces the pathology of vanishing white matter disease in mice. J. Neurosci. 34: 12182–12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone E. A., Thomas J. H., 1994. A screen for nonconditional dauer-constitutive mutations in Caenorhabditis elegans. Genetics 136: 879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurin A. C., Jousse C., Averous J., Parry L., Bruhat A., et al. , 2005. The GCN2 kinase biases feeding behavior to maintain amino acid homeostasis in omnivores. Cell Metab. 1: 273–277. [DOI] [PubMed] [Google Scholar]
- McGrath P. T., Xu Y., Ailion M., Garrison J. L., Butcher R. A., et al. , 2011. Parallel evolution of domesticated Caenorhabditis species targets pheromone receptor genes. Nature 477: 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D., O’Doherty I., Somvanshi R. K., Bethke A., Schroeder F. C., et al. , 2012. Interaction of structure-specific and promiscuous G-protein-coupled receptors mediates small-molecule signaling in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 109: 9917–9922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavitt G. D., Yang W., Hinnebusch A. G., 1997. Homologous segments in three subunits of the guanine nucleotide exchange factor eIF2B mediate translational regulation by phosphorylation of eIF2. Mol. Cell. Biol. 17: 1298–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavitt G. D., Ramaiah K. V., Kimball S. R., Hinnebusch A. G., 1998. eIF2 independently binds two distinct eIF2B subcomplexes that catalyze and regulate guanine-nucleotide exchange. Genes Dev. 12: 514–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottekat A., Becker S., Spencer K. R., Yates J. R., Manning G., et al. , 2013. Insulin biosynthetic interaction network component, TMEM24, facilitates insulin reserve pool release. Cell Rep. 4: 921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pungaliya C., Srinivasan J., Fox B. W., Malik R. U., Ludewig A. H., et al. , 2009. A shortcut to identifying small molecule signals that regulate behavior and development in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 106: 7708–7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran R., Loebbermann J., Nakaya H. I., Khan N., Ma H., et al. , 2016. The amino acid sensor GCN2 controls gut inflammation by inhibiting inflammasome activation. Nature 531: 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll-Mecak A., Alone P., Cao C., Dever T. E., Burley S. K., 2004. X-ray structure of translation initiation factor eIF2γ: implications for tRNA and eIF2α binding. J. Biol. Chem. 279: 10634–10642. [DOI] [PubMed] [Google Scholar]
- Sekine Y., Zyryanova A., Crespillo-Casado A., Fischer P. M., Harding H. P., et al. , 2015. Mutations in a translation initiation factor identify the target of a memory-enhancing compound. Science 348: 1027–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidrauski C., Tsai J. C., Kampmann M., Hearn B. R., Vedantham P., et al. , 2015. Pharmacological dimerization and activation of the exchange factor eIF2B antagonizes the integrated stress response. Elife 4: e07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Hinnebusch A. G., 2009. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136: 731–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern E., Chinnakkaruppan A., David O., Sonenberg N., Rosenblum K., 2013. Blocking the eIF2α kinase (PKR) enhances positive and negative forms of cortex-dependent taste memory. J. Neurosci. 33: 2517–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez de Aldana C. R., Dever T. E., Hinnebusch A. G., 1993. Mutations in the alpha subunit of eukaryotic translation initiation factor 2 (eIF-2 alpha) that overcome the inhibitory effect of eIF-2 alpha phosphorylation on translation initiation. Proc. Natl. Acad. Sci. USA 90: 7215–7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Takeuchi T., Tanaka S., Kubo S. K., Kayo T., et al. , 1999. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J. Clin. Invest. 103: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek R. C., Jiang H. Y., Anthony T. G., 2006. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 34: 7–11. [DOI] [PubMed] [Google Scholar]
- Yang K., Li D. F., Wang X., Liang J., Sitia R., et al. , 2016. Crystal structure of the ERp44-peroxiredoxin 4 complex reveals the molecular mechanisms of thiol-mediated protein retention. Structure 24: 1755–1765. [DOI] [PubMed] [Google Scholar]
- Yang W., Hinnebusch A. G., 1996. Identification of a regulatory subcomplex in the guanine nucleotide exchange factor eIF2B that mediates inhibition by phosphorylated eIF2. Mol. Cell. Biol. 16: 6603–6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains are available upon request. The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.