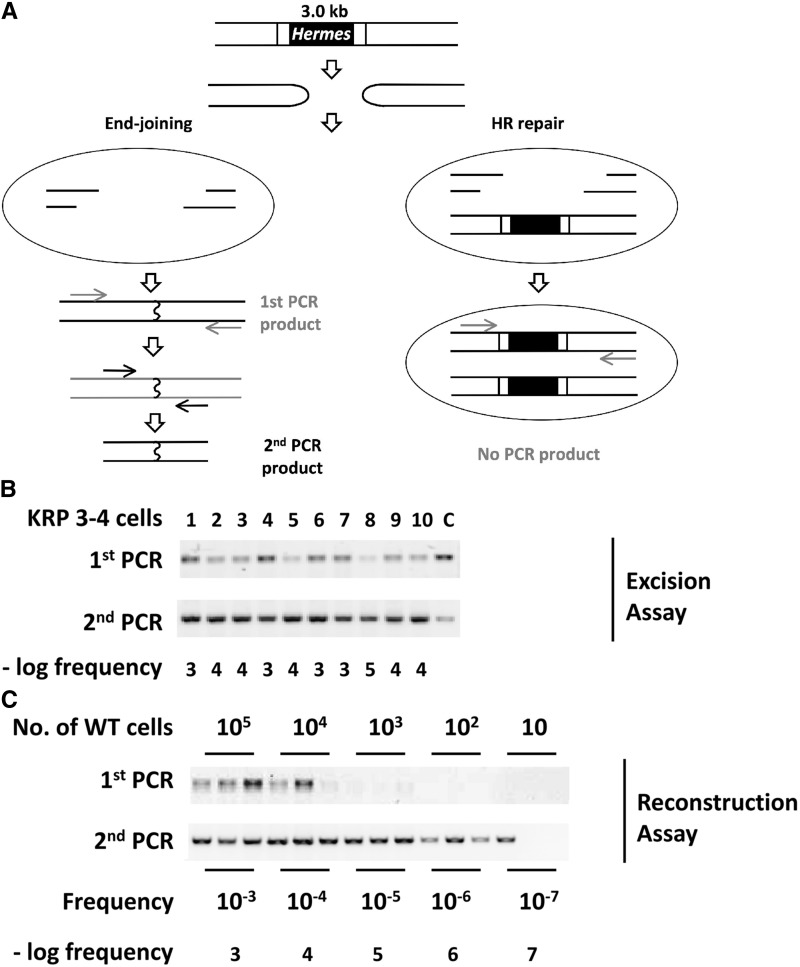
Figure 2.
Removal of a Hermes transposon requires end-joining repair. (A) Transposon excision produces hairpin-capped DSB ends that must be repaired, or cells will lose essential genetic information during mitosis and die. Repair by end-joining (NHEJ or MMEJ, left panel) involves nuclease activities and ligation of the broken ends, which can be identified by PCR. Rare events can be detected by a second PCR with a set of nested primers. Repair by HR in G2 cells that retain an unexcised transposon (right panel) will regenerate the Hermes insertion. This product will not be detected in this assay due to the use of short extension times that do not allow amplification of the Hermes insertion. The arrows indicate primers for the PCR reactions. (B) KRP 3-4 cells containing a single-transposon insertion (Figure S1 in File S1) were transformed with the transposase expression plasmid to generate colonies from single cells. The individual colonies were grown to 5 × 107 cells under conditions that induced transposase expression, and DNA was prepared and used in two rounds of PCR. Excision frequencies were determined by comparison to the standard curve shown in the next panel. The lanes labeled “C” are loaded with various amounts of a marker that show the size of the expected fragment. (C) A reconstruction test was performed to estimate the frequency of Hermes excision. The indicated number of WT, which lack a Hermes insertion, were mixed with 5 × 107 KRP 3-4 cells bearing a transposon and used to prepare DNA. PCR to detect only the excision products (as in A) was performed in triplicate for each sample. Quantitation and comparison of the first and second PCR products indicated that excision frequencies differing by 10-fold could be distinguished over the range of 10−4 to 10−6 per cell. DSB, double-strand breaks; HR, homologous recombination; MMEJ, microhomology-mediated end-joining; NHEJ, nonhomologous end-joining; wild-type, WT.