Abstract
Lis-homology (LisH) motifs are involved in protein dimerization, and the discovery of the conserved N-terminal LisH domain in transducin β-like protein 1 and its receptor (TBL1 and TBLR1) led us to examine the role of this domain in transcriptional repression. Here we show that multiple β-transducin (WD-40) repeat-containing proteins interact to form oligomers in solution and that oligomerization depends on the presence of the LisH domain in each protein. Repression of transcription, as assayed using Gal4 fusion proteins, also depended on the presence of the LisH domain, suggesting that oligomerization is a prerequisite for efficient transcriptional repression. Furthermore, we show that the LisH domain is responsible for the binding to the hypoacetylated histone H4 tail and for stable chromatin targeting by the nuclear receptor corepressor complex. Mutations in conserved residues in the LisH motif of TBL1 and TBLR1 block histone binding, oligomerization, and transcriptional repression, supporting the functional importance of the LisH motif in transcriptional repression. Our results indicate that another WD-40 protein, TBL3, also preferentially binds to the N-terminal domain of TBL1 and TBLR1, and forms oligomers with other WD-40 proteins. Finally, we observed that the WD-40 proteins RbAp46 and RbAp48 of the sin3A corepressor complex failed to dimerize. We also found the specific interaction UbcH/E2 with TBL1, but not RbAp46/48. Altogether, our results thus indicate that the presence of multiple LisH/WD-40 repeat containing proteins is exclusive to nuclear receptor corepressor/ silencing mediator for retinoic and thyroid receptor complexes compared with other class 1 histone deacetylase-containing corepessor complexes.
THE NUCLEAR RECEPTOR corepressor (N-CoR) and the silencing mediator for retinoic and thyroid receptors (SMRT) were identified initially as corepressors for nuclear receptors such as thyroid hormone receptors (TRs) and retinoic acid receptors (1, 2). These proteins are, in turn, repressed by many other transcription factors including Mad/Mxi, BCL6/LAZ3, ETO, and CBF (3). Recent efforts in biochemical purification and characterization of both SMRT and N-CoR demonstrated that they exist as large protein complexes and are associated primarily with histone deacetylase (HDAC)3 (4, 5, 8). Consistent with the biochemical results that HDAC3 is the only HDAC identified in the purified complexes, knock down of HDAC3 using small interference RNA impaired repression by unliganded TR (6, 7). In addition to HDAC3, the purified SMRT complex also contained transducin β-like 1 (TBL1) (4). Purification of the N-CoR complex by Zhang et al. (8) identified two additional N-CoR/SMRT-associated proteins, GPS2, a protein involved in intracellular signaling, and transducin β-like 1 receptor (TBLR1).
TBL1/TBLR1, complexed with SMRT and N-CoR, stabilizes the quaternary structure of the corepressor assemblage through additional contacts with HDAC3 and bind to histones H2B and H4 to assist in chromatin substrate recognition (4, 7, 8, 9). TBL1 is a LisH (Lis1 homology domain)/WD-40-containing protein, originally associated with an X-linked human disorder in which a microdeletion of the C-terminal part of the Tbl1 gene was suggested to be responsible for the hearing defect (10, 11). Mutations in the fly ortholog, Ebi, affect multiple processes including epidermal growth factor receptor-mediated neuronal differentiation (12). SET3 is a SMRT/N-CoR homologous complex observed in yeast (13), which illustrates the conservation of such complexes across eukaryotic species. More recently, TBL1 and TBLR1 were observed to selectively serve as mediators of the required exchange of the nuclear receptor corepressors, N-CoR/SMRT, for coactivators upon ligand binding/stimulation (14).
TBL1 homologs appear to be widespread in eukaryotes with Sif2p from yeasts constituting a predicted TBL1 homolog based on the fact that it contains C-terminal WD-40 repeats and an N-terminal LisH domain and functions as a corepressor in conjunction with other DNA-binding repressors. The LisH domain of Sif2p mediates tetramerization and interaction with components of the SET3C corepressor complex (15). The Drosophilia Gro protein, another TBL1 homolog, contains multiple WD-40 repeats in the C terminus and forms a homotetramer through its N-terminal domain (16, 17). More than 100 eukaryotic proteins contain the LisH motif including: Lis1, Sif2p, TBL1, TBLR1, OFD1, and p220NPAT (15, 18, 19). TBL1 and its homologs from multicellular species share a highly conserved N-terminal LisH domain and a C-terminal WD-40 repeat domain. Previous studies have suggested that the N-terminal region of TBL1/TBLR1 can function as a transcriptional repressor, but that function has not yet been clearly demonstrated.
Here we present evidence for a novel function of the LisH domain in feed-forward transcriptional repression mediated by SMRT/N-CoR corepressor complexes in chromatin. Furthermore, we show that the presence of multiple LisH/WD-40 repeat containing proteins is unique to N-CoR/SMRT corepressor complexes compared with other class 1 HDAC-containing complexes.
RESULTS
TBL1/TBLR1 Oligomerization Is Mediated by LisH Domains
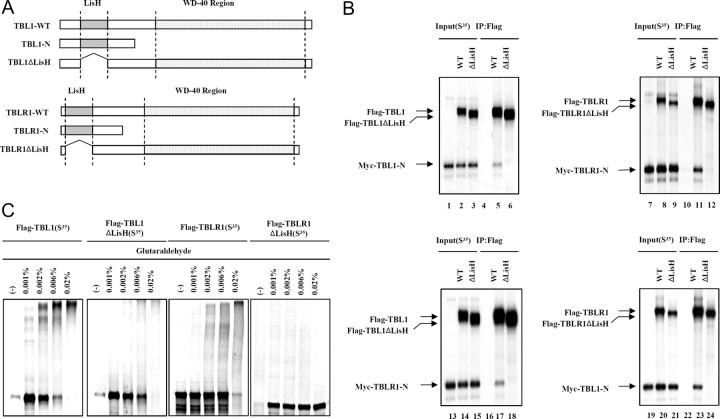
Recently, LisH domains have been shown to function in protein dimerization (19, 20). To investigate the role of the LisH domain in TBL1/TBLR1 interaction, we first sought to determine whether TBL1 interacts with itself or with TBLR1 through the LisH domain. Myc-tagged versions of the N terminus-containing LisH domain of TBL1 and TBLR1 (Myc-TBL1-N and Myc-TBLR1-N) were generated to perform coimmunoprecipitation assays (Fig. 1A) in comparison with wild-type, full-length Flag-tagged TBL1 and TBLR1, and LisH domain-deficient forms of TBL1 and TBLR1 (TBL1ΔLisH and TBLR1ΔLisH). The three forms of TBL1 and TBLR1 were [35S]methionine labeled, either together or separately as indicated, and then immunoprecipitated with Flag-agarose M2 beads. When expressed separately, Myc-TBL1-N and Myc-TBLR1-N did not bind M2 beads (Fig. 1B, lanes 4 and 10). However, Myc-TBL1-N and Myc-TBLR1-N did bind to the beads when cotranslated with Flag-TBL1 and Flag-TBLR1, respectively (Fig. 1B. lanes 5 and 11), indicating the formation of dimers through the N-terminal domain. In contrast, Flag-TBL1ΔLisH and TBLR1ΔLisH did not interact with Myc-TBL1-N and Myc-TBLR1-N (Fig. 1B, lanes 6 and 12). These results show that TBL1 and TBLR1 were required for LisH domain homodimer formation. Next, we examined the potential for heterodimerization of full-length Flag-TBL1 and Myc-TBLR1-N, or full-length Flag-TBLR1 and Myc-TBL1-N. As hypothesized, TBL1 interacts with TBLR1 through the LisH domain (Fig. 1B, lanes 17 and 23). Altogether, these results confirmed that the LisH domain is required for the heterodimerization of TBL1 and TBLR1.
Fig. 1.
An N-Terminal LisH Domain in TBL1/TBLR1 Is Required for Oligomerization
A, Schematic of various full-length or truncated [35S]methionine-labeled TBL1 and TBLR1 proteins that were produced by in vitro translation. B, In vitro coimmunomobilization assays. A Myc-tagged TBL1 N-terminal region containing LisH (Myc-TBL1-N) was in vitro cotranslated with Flag-tagged, wild-type, full-length TBL1 (WT) and TBL1 without LisH (TBL1ΔLisH) or translated alone (left upper panel). A Myc-tagged TBLR1 N-terminal region containing LisH (Myc-TBLR1-N) was in vitro cotranslated with Flag-tagged, wild-type, full-length TBLR1 (WT) and TBLR1ΔLis-H (TBLR1ΔLis-H) or translated alone (right upper panel). The Myc-tagged TBLR1 N-terminal region containing the LisH domain (Myc-TBLR1-N) was in vitro cotranslated with Flag-tagged, wild-type, full-length TBL1 (WT) and TBL1ΔLisH or translated alone (left lower panel). The Myc-tagged TBL1 N-terminal region containing the LisH domain (Myc-TBL1-N) was in vitro cotranslated with Flag-tagged, wild-type, full-length TBLR1 (WT) and TBLR1ΔLisH or translated alone (right lower panel). The translation products were incubated with Flag M2-agarose beads. After extensive washing, Flag-bound proteins were eluted and analyzed by SDS-PAGE and autoradiography. C, Equal amounts of [35S]methionine-labeled proteins plus DTT were incubated with various concentrations of glutaraldehyde (indicated above each lane). Cross-linked products were resolved by SDS-PAGE and visualized by Coomassie blue staining and analyzed by autoradiography. IP, Immunoprecipitation.
Whereas Fig. 1B shows heterodimerization between TBL1 and TBLR1, it is not clear whether the binding of TBL1 and TBLR1 is indicative of oligomerization between these proteins. To further investigate this, we employed glutaraldehyde cross-linking analysis to examine the oligomeric state of TBL1 and TBLR1. Protein cross-linking assays were carried out to confirm that the LisH domains of TBL1 and TBLR1 mediate protein oligomerization. In vitro translated [35S]methionine-labeled proteins were incubated with concentrations of glutaraldehyde ranging from 0.001 to 0.02% and then analyzed by SDS-PAGE and autoradiography. After treatment with 0.001% glutaraldehyde, we observed the higher-molecular mass species with masses comparable to those expected for oligomers of wild-type TBL1 and TBLR1 (Fig. 1C, first and third panel, lanes 3 and 4, respectively). At higher concentrations of glutaraldehyde, both wild-type TBL1 and TBLR1 were stoichiometrically cross-linked into a high-molecular mass form (Fig. 1C, first and third panel, lane 5, respectively). In contrast to wild-type proteins, [35S]methionine-labeled TBL1ΔLisH or TBLR1ΔLisH did not yield cross-linked species under the same conditions (Fig. 1C, second and forth panel). Altogether, these results confirmed that the LisH domain is necessary for homooligomerization of both TBL1 and TBLR1.
Role of LisH in Transcriptional Repression
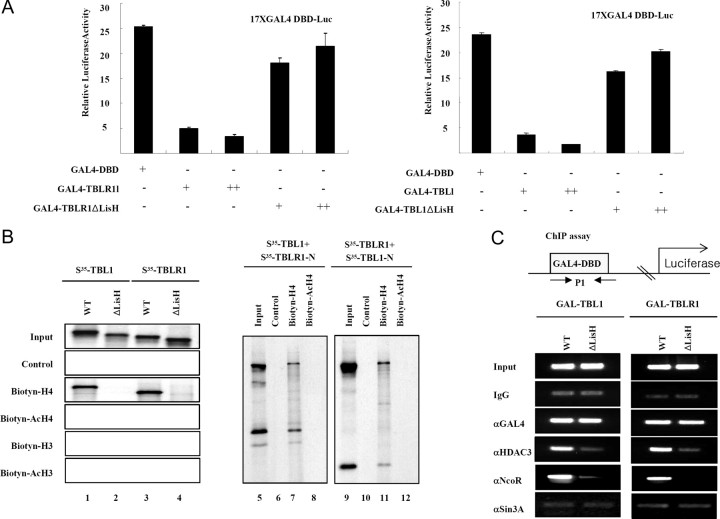
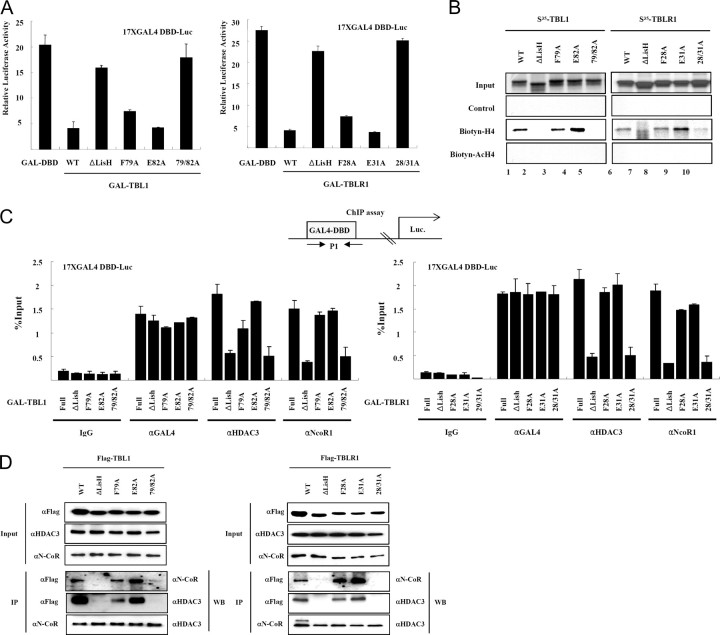
Previous studies revealed repression domains present in the N-terminal regions of TBL1 and TBLR1. The ability of N-terminal LisH domains in both TBL1 and TBLR1 to facilitate oligomerization prompted us to test whether LisH is correlated with transcriptional repression. As shown in Fig. 2, GAL4 DNA-binding domain fusion proteins of TBL1 and TBLR1 could effectively repress a thymidine kinase-luciferase reporter bearing GAL4-binding sites in transient transfection assays. The repression activity of TBL1 appears to be dependent on the presence of LisH because expression of TBL1ΔLisH or TBLR1ΔLisH failed to repress transcription. These data indicated that the LisH domains present in TBL1 and TBLR1 are required for transcriptional repression.
Fig. 2.
The Recognition of Hypoacetylated Histone H4 Tail by the LisH Domain Is Required for N-CoR-Mediated Transcriptional Repression
A, 293T cells were transfected with a GAL4 binding site-driven luciferase reporter plasmid in addition to the indicated expression plasmids. The fold repression shown is relative to the luciferase activity obtained by simian virus 40 cotransfection. Results are presented as the means of two independent experiments performed in triplicate. B, Biotinylated-histone H3, acetylated histone H3, histone H4, and acetylated histone H4 N-terminal tail peptide were first immobilized on streptavidin agarose beads and then incubated with in vitro translated material labeled with [35S]methionine Flag-TBL1, TBLR1, TBL1ΔLisH, and TBLR1ΔLisH, respectively (left panel). Flag-TBL1 was in vitro cotranslated with Flag-TBL1-N or Flag-TBLR1-N. The translation products were incubated with H4 N-terminal tail peptide-conjugated streptavidin agarose beads. After extensive washing, the bound proteins were eluted and analyzed by SDS-PAGE and autoradiography (right panel). C, HeLa cells were transfected with each Gal fusion protein construct and the luciferase reporter, and ChIP assays were carried out. DBD, DNA-binding domain; WT, wild type.
We recently showed that TBL1 and TBLR1 are essential for targeting of the N-CoR and SMRT complexes to the TR target gene deiodinase 1 (D1) through reorganization and binding to the deacetylated histone H4 tail product (21). Thus, we hypothesized that LisH of TBL1 and TBLR1 may be involved in targeting N-CoR and SMRT complexes to chromatin via histone binding and corepressor complex subunit binding. To test this hypothesis we examined the interaction of the LisH domains in TBL1/TBLR1 with histones. Because acetylation of H4 has a more pronounced effect on TBL1 binding, we focused on this histone. We first compared the binding of TBL1, TBL1ΔLisH, TBLR1, and TBLR1ΔLisH with the chemically synthesized histone H4 tail peptide (amino acids 1–30) without (H4) or with acetylation at lysines 5, 8, 12, and 16 (acH4). To facilitate the pull-down assay, a biotin residue was linked to the C terminus of each peptide through a short linker (Gly-Gly-Lys) sequence. These peptides were immobilized to streptavidin agarose beads and used to pull down in vitro translated, [35S]methionine labeled, TBL1, TBL1ΔLisH, TBLR1, and TBLR1ΔLisH. Consistent with previous observations (21), the wild-type, full-length TBL1 and TBLR1 proteins bound to hypoacetylated histone H4, but not H3 (Fig. 2B, lanes 1 and 3). Interestingly, both TBL1ΔLisH and TBLR1ΔLisH, which lack LisH, failed to interact with hypoacetylated histone H4 tail (Fig. 2B, lanes 2 and 4), indicating LisH-dependent histone binding. Additionally, heterodimers of TBL1 and TBLR1 efficiently bound hypoacetylated histone H4 tail through the LisH domain (Fig. 2B, lanes 7 and 11), implying interaction between oligomeric TBL1/TBLR1 and the hypoacetylated histone tail that is found in chromatin.
Finally, using a chromatin immunoprecipitation (ChIP) assay, we found that GAL-TBL1 recruited the N-CoR and HDAC3, subunits of the N-CoR complex, but not sin3A to the promoter of the transfected reporter (Fig. 2C). In contrast, GAL4-TBL1ΔLisH failed to recruit these proteins. Thus, these experiments provide evidence that histone binding and oligomerization by LisH in TBL1 and TBLR1 are essential for stable chromatin targeting by the N-CoR complex.
LisH Stabilizes the N-CoR/SMRT Complex
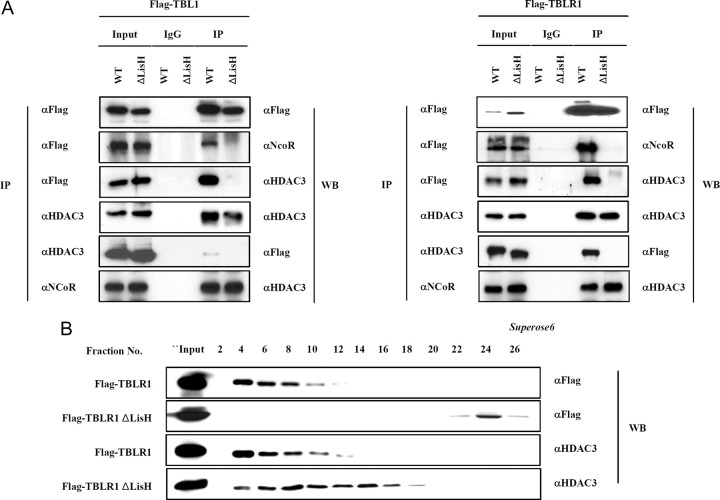
Because two distinct domains of TBL1 and TBLR1 were reported to interact with two different regions of N-CoR (23), we examined the possibility that WD-40 repeats in the C-terminal of TBL1/TBLR1 alone are insufficient for interaction with N-CoR. Expression constructs encoding Flag-tagged TBL1, TBL1ΔLisH, Flag-tagged TBLR1, and TBLR1ΔLisH were each transfected into HeLa cells. Subsequent immunoprecipitation experiments using flag M2 beads were performed to test binding to endogenous N-CoR and HDAC3. As shown in Fig. 3A, the wild-type, full-length Flag-TBL1 and Flag-TBLR1 interact with N-CoR and HDAC3, but Flag-TBL1ΔLisH and Flag-TBLR1ΔLisH do not. Although there was evidence for the direct interaction between N-CoR and WD-40 repeats present in TBL1/TBLR1, the TBL1/TBLR1 mutants lacking LisH fail to form a stable interaction with the N-CoR-HDAC3 complex. To substantiate this result, we analyzed the formation of N-CoR complexes by gel filtration. Flag-tagged TBLR1 or TBLR1ΔLisH constructs were transfected into HeLa cells, and transfected cell extracts were fractionated using a Superose 6 gel filtration column. The concentrated fractions were analyzed by SDS-PAGE and Western blot analysis. Flag-TBLR1 wild-type protein migrated with HDAC3 as expected (Fig. 3B). In contrast, the TBLR1ΔLisH protein failed to incorporate into the HDAC3 corepressor complex, a result that was consistent with results from coimmunoprecipitation assays (Fig. 3B). Furthermore, the molecular weight of the HDAC3 corepressor complex was lower when TBLR1ΔLisH was overexpressed (Fig. 3B). These results emphasized the functional importance of the LisH domain present in the TBL1 and TBLR1 proteins in the formation of a stable N-CoR/SMRT corepressor complex.
Fig. 3.
The Stabilization of N-CoR/SMRT Corepressor Complexes by LisH Domain
A, Flag-tagged TBL1, TBLR1, TBL1ΔLisH, and TBLR1ΔLisH constructs were transfected into HeLa cells. Immunoprecipitation was performed (left), and the precipitated materials were analyzed by Western blot with antibodies to the specific proteins listed on the right. B, HeLa nuclear extracts transfected with the indicated plasmids (Flag-tagged TBLR1 and TBLR1ΔLisH) were separated by Superose 6 gel filtration, and fractions were analyzed by Western blot with antibodies to the specific proteins indicated on the right. IP, Immunoprecipitation; WB, Western blot.
Mutation Analysis of the LisH Domain
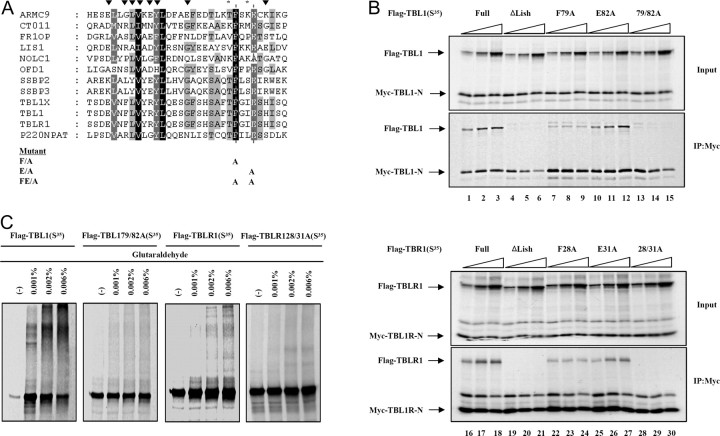
The role of the Lis-H domain in TBL1/TBLR1-mediated transcriptional repression led us to further define the functional aspects of this domain in great detail. More than 100 eukaryotic proteins contain LisH, including Lis1, WDR47, and p220NPAT (18, 22). Sequence analysis revealed that the LisH domain is approximately 27–34 amino acids long and is characterized by hydrophobic residues at positions 5, 8, 9, 10, 12, 13, 18, 25, and 31, which are highly conserved across various human proteins (Fig. 4A). In the case of p220NPAT, mutating Phe27 resulted in a modest decrease in transcriptional activity. However, the transcriptional activity of p220NPAT was totally reduced when both Phe27 and the highly conserved Glu30 residue were mutated to Ala. Therefore, we produced mutants of Phe79 and/or Glu82 in TBL1 and of Phe28 and/or Glu31 in TBLR1, which correspond to Phe27 and Glu30 in p220NPAT (each to Ala) (Fig. 4A). The Flag-tagged TBL1 F79A, E82A, and 79/82A were in vitro cotranslated with Myc-tagged TBL1-N, containing LisH. The results showed that TBL1 F72A still formed LisH-dependent interactions, although the level of binding appeared to be reduced compared with wild-type TBL1. On the other hand, TBL1 E82A behaved similarly to the wild-type TBL1 (Fig. 4B, lanes 10 and 11). Like the ΔLisH mutant, the dual point mutation of both F79A and E82A failed to interact with the N terminus of TBL1 (Fig. 4B, lanes 9 and 12). In agreement with the above result, mutations of both F28A and E31A in TBLR1 resulted in loss of binding to the TBLR1 N terminus, suggesting that these two amino acids are critical to the function of LisH (Fig. 4B, lane 24). In addition, protein cross-linking assays further confirmed that these dual point mutations in TBL1 and TBLR1 eliminate residues necessary for TBL1 and TBLR1 oligomerization (Fig. 4C).
Fig. 4.
Sequence Analysis and Mutagenesis of the LisH Domain
A, Clustal analysis of LisH motifs from 12 human proteins is shown with highly conserved residues marked by ▾. Alignments were generated using Clustal W (http://www.ebi.ac.uk/clustalw/) and displayed using Jalview (http://www2.ebi.ac.uk/∼michele/jalview/contents.htlm). Point mutation constructs of the LisH domain of TBL1 and TBLR1 are shown with asterisks indicating mutation sites. B, The Myc-tagged TBL1 or TBLR1 N-terminal region containing the LisH domain (Myc-TBL1-N or TBLR1-N) was in vitro cotranslated with increasing amounts of wild-type Flag-TBL1/TBLR1 or mutant proteins (100, 200, 400 ng) in the presence of [35S]methionine. The translation products were incubated with Flag M2-agarose beads. After extensive washing, Flag-bound proteins were eluted and analyzed by SDS-PAGE and autoradiography. C, Equal amounts of [35S]methionine-labeled proteins plus DTT were incubated with various concentrations of glutaraldehyde (indicated above each lane). Cross-linked products were resolved by SDS-PAGE and visualized by Coomassie blue staining and analyzed by autoradiography. IP, Immunoprecipitation.
To test whether F28 and E31 are required for transcriptional repression, an in vivo transcription assay with a Gal4-binding site-containing reporter was carried out. Gal4-TBL1 F79/E82A fusion protein caused the loss of repression, whereas GAL4-F79A and GAL4-E82A repressed transcription as well as wild type (Fig. 5A). In addition, the double mutant (F79A, E82A) protein lost the ability to interact with hypoacetylated histone H4 tail peptide just as the ΔLisH protein did (Fig. 5B, lanes 5 and 10). By chromatin targeting, we confirmed that GAL-TBL1 F79/E82A and GAL-TBLR1 F28/E31A proteins failed to recruit any corepressor proteins (Fig. 5C, lanes 4 and 8). Finally, the coimmunoprecipitation experiments clearly demonstrated that the Flag-TBL1 F79/E82A protein could not interact with N-CoR and HDAC3, but the F79A and E82A individual mutants associated with N-CoR through two interaction domains (Fig. 5D). The mutant forms of TBLR1 gave results similar to those obtained from the TBLl mutants (Fig. 5). Collectively, these experiments provide evidence that two amino acids in the N terminus of TBL1 and TBLR1 are essential for LisH function.
Fig. 5.
Identification of Amino Acids Essential for the Function of the LisH Domain
A, 293T cells were transfected with a GAL4 binding site-driven luciferase reporter plasmid in addition to the indicated expression plasmids. The fold repression shown is relative to the luciferase activity obtained by simian virus 40 cotransfection. Results are shown as the means of two independent experiments performed in triplicate. B, Biotinylated histone H3, acetylated histone H3, histone H4, and acetylated histone H4 N-terminal tail peptide were first immobilized on streptavidin agarose beads, and then incubated with in vitro-translated [35S]methionine-labeled point mutant proteins. The translation products were incubated with H4 N-terminal tail peptide-conjugated streptavidin agarose beads, and after extensive washing, the bound proteins were eluted and analyzed by SDS-PAGE and autoradiography. C, HeLa cells were cotransfected with each Gal fusion protein construct and the luciferase reporter construct, and ChIP assays were carried out. The results were analyzed by real-time PCR and were shown as the percentage of input. The results are shown as means ± sd calculated from three independent experiments. D, Flag-tagged protein constructs were transfected into HeLa cells. Immunoprecipitation was performed with antibodies against specific proteins indicated on the left, and the precipitated material was analyzed by Western blot with antibodies to proteins (shown on the right). DBD, DNA-binding domain; WB, Western blot; WT, wild type.
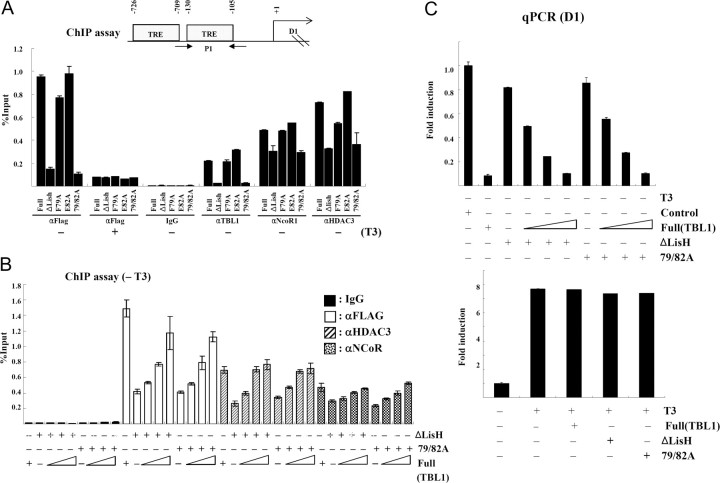
Stabilization by LisH Is Required for Chromatin Targeting of N-CoR Corepressor Complex to the D1 Promoter
Coimmunoprecipitation and gel-filtration analysis showed that the LisH domain stabilized the N-CoR/SMRT corepressor complex. To unravel the roles of LisH domain in chromatin targeting of N-CoR corepressor complex, we first sought to investigate the effect of destabilization of the N-CoR corepressor complex on targeting to the endogenous TR target gene, D1, in a HeLa α2 cell line that constitutively expresses a FLAG-tagged human TRα (7). The D1 gene promoter contains a well-characterized thyroid hormone response element located at positions −105 to −130 relative to the transcriptional start site (Fig. 6A). In HeLa α2 cells, this gene is actively repressed by TRα in the absence of T3 and activated in the presence of T3 (7). ChIP assays showed that FLAG-TBL1 and TBLR1 had little effect on the recruitment of N-CoR and HDAC3 to the D1 promoter. Interestingly, overexpressed TBL1ΔLisH and TBLR1ΔLisH inhibited chromatin targeting by both N-CoR and HDAC3. Like the ΔLisH mutant, the F79A and E82A point mutations also reduced the recruitment of N-CoR corepressor complex to the D1 promoter (Fig. 6A). Because ΔLisH could still interact with another component of the N-CoR corepressor complex, we decided to test whether an excessive amount of wild-type TBL1 could overcome the dominant-negative effect induced by ΔLisH. As shown in Fig. 6B, chromatin targeting of the N-CoR corepressor complex was recovered when wild-type TBL1 was overexpressed in a dose-dependent manner. In agreement with this result, the D1 gene expression, which was decreased by ΔLisH, was repressed by the addition of wild-type TBL1 (Fig. 6C). These data suggest that TBL1 and TBLR1 stabilize chromatin targeting by the corepressor complex through the LisH domain.
Fig. 6.
Stabilization by LisH Domain Is Required for Chromatin Targeting of N-CoR Corepressor Complex to the D1 Promoter
A, Flag-tagged TBL1, TBLR1, TBL1ΔLisH, and TBLR1ΔLisH constructs were transfected into HeLa α2 cells, and ChIP assays were carried out. The results were analyzed by real-time PCR and were shown as the percentage of input. The results are shown as means ± sd calculated from three independent experiments. B, Flag-tagged TBL1ΔLisH and Flag-TBL1 79/82A constructs were cotransfected with increasing amounts of wild-type Flag-TBL1 (2, 4, 8 μg) in to HeLa α2 cells, and ChIP assays were carried out. C, HeLa α2 cells were transfected with Flag-TBL1 and/or TBL1ΔLisH as indicated. Three days after treatment, total RNAs were prepared and processed for real-time PCR analysis using gene-specific primers. The results are shown as means ± sd calculated from three independent experiments. qPCR, quantitative PCR; TRE, thyroid hormone response element.
Multiple LisH- and WD40 Repeat-Containing Proteins Are Essential for Feed-Forward Repression Mediated by N-CoR/SMRT Corepressor Complexes
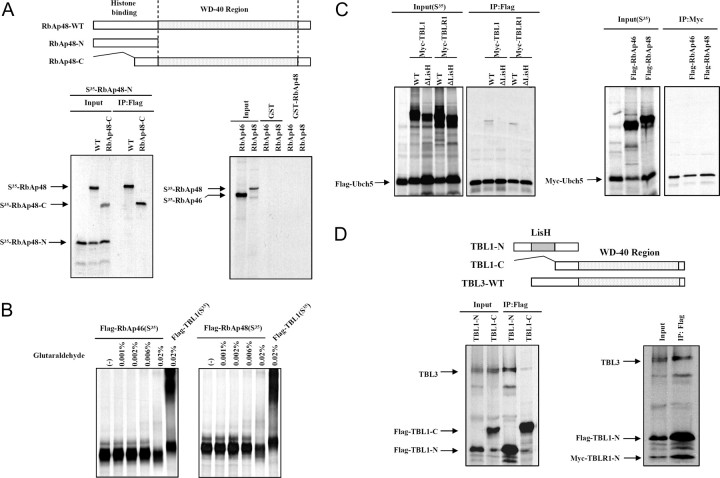
Because the RbAp46 and RbAp48 proteins in Sin3 and Mi-2/NuRD complexes are analogous to TBL1 and TBLR1 proteins in SMRT/N-CoR complexes, we proceeded to tested whether the oligomerization by histone-binding proteins is a general mechanism of action of class1 HDAC-containing corepressor complexes. The Flag-tagged C-terminal domain of RbAp48 (RbAp48-C); the wild-type, full-length Flag-tagged RbAp46 and RbAp48 proteins; and a WD-40 repeat domain-deleted form of RbAp48 (RbAp48-N) were generated to perform coimmunoprecipitation assays. The three forms of RbAp46 and RbAp48 were [35S]methionine labeled together, or separately, as indicated through in vitro translation and immunoprecipitated with Flag-agarose M2 beads. As shown in Fig. 7A, RbAp48 did not interact with either RbAp48-N or itself. Furthermore, glutathione-S-transferase (GST)-pull down experiments showed that RbAp46 failed to bind RbAp48. To further confirm this result, we employed protein cross-linking assays. In vitro translated [35S]methionine-labeled proteins were incubated with concentrations of glutaraldehyde (0.001–0.02%) and analyzed by SDS-PAGE and autoradiography. In contrast to TBL1 and TBLR1, [35S]methionine-labeled RbAp46 or RbAp48 did not yield cross-linked species under the same conditions (Fig. 7B). These results suggest that oligomerization of histone binding proteins is not a common mechanism across all class 1 HDAC-containing corepressor complexes.
Fig. 7.
Multiple LisH/WD-40 Repeat-Containing Proteins Are Found in N-CoR/SMRT Corepressor Complexes, But Not the sin3A Corepressor Complex
A, Flag-tagged RbAP48 (Flag-RbAp48) and RbAp48-C C-terminal region (Flag-RbAp48-C) were in vitro cotranslated with the Myc-tagged RbAp48 N-terminal region containing the histone-binding domain (Myc-RbAp48-N). The translation products were incubated with Flag M2-agarose beads, and after extensive washing, Flag-bound proteins were eluted and analyzed by SDS-PAGE and autoradiography (left panel). For the GST-pull down assay, each fragment was in vitro translated, [35S]methionine labeled, and used for in vitro pull-down assays using GST-RbAp46 and GST-RbAp48 fusion proteins. Input was 20% of the sample used for pull-down assays (right panel). B, Equal amounts of [35S]methionine-labeled proteins plus DTT were incubated with various concentrations of glutaraldehyde (indicated above each lane). Cross-linked products were resolved by SDS-PAGE and visualized by Coomassie blue staining and analyzed by autoradiography. C, The in vitro cotranslation products were incubated with Flag M2-agarose beads, and after extensive washing, Flag-bound proteins were eluted and analyzed by SDS-PAGE and autoradiography. D, Flag-TBL1-N and Flag-TBL1-C were in vitro cotranslated with Myc-TBL3. The translation products were incubated with Flag M2-agarose beads, and after extensive washing, Flag-bound proteins were eluted and analyzed by SDS-PAGE and autoradiography. IP, Immunoprecipitation; WT, wild type.
TBL1/TBLR1 are known to be specific adaptors for the recruitment of the ubiquitin-conjugating/19S proteasome complex (14), with TBLR1 selectively serving to mediate required exchanges of corepressor for coactivators upon ligand binding. We examined whether the exchanges of the corepressor for coactivators upon ligand binding through LisH proteins is unique to N-CoR and SMRT corepressor complexes. Expression constructs encoding Flag-tagged TBLR1ΔLisH, Flag-tagged TBLR1, TBL1, TBLR1ΔLisH, RbAp46, and RbAp48 were in vitro cotranslated with Myc-tagged UbcH9. Subsequent immunoprecipitation experiments using Flag M2 beads showed a LisH-dependent interaction between TBL1/TBLR1 and UbcH9 (Fig. 7C). On the other hand, both RbAp46 and RbAP48 failed to interact with UbcH9, indicating that corepressor/coactivator exchange by TBL1 and TBLR1 is exclusive to N-CoR and SMRT corepressor complexes.
Recently we observed the presence of an additional WD-40 repeat-containing protein, TBL3, in the N-CoR corepressor complex. Thus, we tested whether TBL3 interacts with TBL1 and/or TBLR1. TBL3 preferentially binds to the N-terminal domain, but not C-terminal domain, of TBL1 just as TBLR1 does (Fig. 7D). Furthermore, three WD-40 repeat-containing proteins efficiently interacted with each other after cotranslation, indicating that the presence of multiple LisH- and WD-40 repeat-containing proteins in a complex is unique to N-CoR/SMRT corepressor complexes compared with other HDAC-containing corepressor complexes.
DISCUSSION
The LisH motif is present in many human proteins including Lis1, p220NPAT, Sif2p, TBL1, and TBLR1, where it is typically 33 amino acids long and is characterized by the presence of hydrophobic residues. Up to this point, the only biological function assigned to this motif was based on its ability to facilitate protein oligomerization. A recent study revealed that Sif2p exists as a tetramer, and that the N-terminal LisH-containing domain mediates tetramerization as well as the interactions with the SET3C protein complex. Because Sif2p appears to be the yeast homolog of human TBL1 and TBLR1, which are components of the N-CoR/SMRT complexes, structural and oligomeric properties are likely to be very similar between such complexes.
The N-terminal LisH domains of TBL1 and TBLR1 bind hypoacetylated histone H4, shown for the first time in this study. Using in vitro pull-down assays with synthetic histone tail peptide, we found that the wild-type, full-length TBL1 and TBLR1 proteins bind specifically to hypoacetylated histone H4, which was consistent with observations from previous studies. TBL1 and TBLR1 proteins lacking LisH failed to interact with hypoacetylated histone H4 tail, indicating that this domain is required for histone binding. Furthermore, as shown by ChIP assay, we confirmed that the loss of histone binding induced by the deletion of LisH led to the dissociation of the N-CoR complex from chromatin. The binding of TBL1 and TBLR1 to hypoacetylated histones was shown to be required for stable targeting of the SMRT/N-CoR corepressor complex to chromatin. Thus, LisH-mediated oligomerization and histone binding are required for efficient transcriptional repression.
Previously, two domains of TBL1 and TBLR1 were observed to interact with two different regions of N-CoR (7). The N-terminal domains of TBL1 and TBLR1 interact with N-CoR RD1, whereas the first three WD-40 repeats in the C-terminal domain interact with N-CoR residues 1801–1965. Chromatin targeting by either TBL1 or TBLR1 was disrupted by the deletion of LisH even though the WD-40 repeats in the C-terminal domain are predicted to interact with N-CoR. Wild-type, full-length TBL1 and TBLR1 interact with N-CoR and HDAC3, but this was not observed with TBL1 and TBLR1 mutants, which lack Lis-H. Interestingly, although there was credible evidence for the direct interaction between N-CoR and WD-40 repeats of TBL1/TBLR1, both TBL1 and TBLR1 that lack LisH fail to associate with the N-CoR-HDAC3 complex. Moreover, the molecular weight of the HDAC3 corepressor complex was lower when the TBLR1 LisH mutant was overexpressed. The ChIP assays on D1 promoter clearly demonstrated that the stabilization by LisH domain is required for chromatin targeting by the N-CoR corepressor complex.
A common feature of all three major mammalian class I HDAC-containing complexes (Sin3A, NURD, and SMRT/N-CoR) is the presence of two highly related WD-40 repeat proteins (RbAp46/48 in Sin3A and NURD and TBL1/TBLR1 in SMRT/N-CoR). The association of WD-40 repeat proteins with HDACs is conserved across eukaryotes (1). For these proteins, transcriptional repression correlates with histone binding (1, 23, 24). One well-known TBL1/TBLR1 homolog in yeast is Tup1. Jabet et al. (25) showed that the N-terminal domain is primarily helical in structure and forms a stable tetramerization domain, which is named the Tup1 coiled coil domain. The arrangement of four Tup1 domains for every Ssn6p unit results in a stable repression complex through the wrapping of hypoacetylated histone tails, and transcriptional repression activity correlates with histone binding (26). The LisH domain present in TBL1 and TBLR1 could also fulfill a similar function, and mechanistic similarities between Tup1- and TBL1/TBLR1-mediated repression may exist. More recently, TBL1 and TBLR1 were shown to be required for feed-forward repression by SMRT/N-CoR corepressor complexes (21) and that targeting SMRT/N-CoR complexes to the deiodinase 1 gene requires the interaction between TBL1/TBLR1 and hypoacetylated histones. Our present data further support a feed-forward model for targeting SMRT/N-CoR complexes to chromatin.
RbAp46 was reported to bind preferentially to hypoacetylated histones in vitro (27), which was considered to be a conserved function among WD-40 repeat proteins present in various corepressor complexes. However, RbAp48 did not interact with either RbAp48-N or with itself. Protein cross-linking analysis showed that RbAp46 failed to form oligomers. TBL1/TBLR1 are known to be specific adaptors for the recruitment of the ubiquitin-conjugating/19S proteasome complex, with TBLR1 selectively serving to mediate a required exchange of corepressors for coactivators upon ligand binding. As expected, we observed the specific interaction of UbcH/E2 enzyme with TBL1 through LisH, but not RbAp46/48. Additionally, we observed the presence of an additional WD-40 repeat containing protein, TBL3, in the N-CoR corepressor complex. TBL3 preferentially binds to the N-terminal domain of TBL1. TBLR1, in turn, forms oligomers with other WD-40 repeat proteins. We propose that the presence of multiple LisH domain-containing proteins is not consistent across all class1 HDAC-containing corepressor complexes.
We present evidence for a novel function of the LisH domain in feed-forward transcriptional repression mediated through the SMRT/N-CoR corepressor complex. We have found that WD-40 repeat-containing proteins, TBL1 and TBLR1, exist as oligomers in solution and that oligomerization depends on the LisH domain, which is conserved in the N-terminal region of these proteins. Furthermore, we show that LisH is responsible for binding to hypoacetylated histone. We assert that the primary function of the LisH domain is to mediate oligomerization and histone binding. Finally, we show the presence of multiple LisH/WD-40 repeat-containing proteins in N-CoR/SMRT corepressor complexes, but not in sin3A corepressor complexes.
MATERIALS AND METHODS
Cell Culture and DNA Cloning
The wild-type, full-length TBL1/TBLR1 and LisH domain-deficient constructs TBL1ΔLisH/TBLR1ΔLisH were generated by PCR and cloned into two plasmid vectors: pSG5-KF2M1 (Sigma Chemical Co., St. Louis, MO) and pCMV-GAL4. TBL1-N and TBLR1-N were made by PCR and subcloned into plasmids pGBKT7-GAL-Myc (CLONTECH Laboratories, Inc., Mountain View, CA) and pSG5-KM2M1 (Sigma). The various mutants of TBL1 and TBLR1 were created using the QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA.). The plasmids for reporter gene assays (GAL4-DBD, 17XGAL4-Tk-Luc, and pSV40) were described previously (7). For GST-fusion proteins, RbAp48 was created by PCR by using a cDNA clone, followed by cloning into the pGEX4T-1 GST expression vector (Amersham Pharmacia Biotech, Piscataway, NJ). The construct for GST-RbAp48 was expressed in Escherichia coli BL21 strain and affinity purified using glutathione-Sepharose 4B beads (Amersham Pharmacia Biotech) following the manufacturer’s protocol. The TBL3 and RbAp48 clones were acquired from 21C Human Frontier Human Gene Bank (Daejeon, Korea) and confirmed by sequencing. Cell lines used in this study include HeLa α2 and HeLa. All cell lines were grown at 37 C under 5% CO2 in DMEM (Life Technologies, Inc., Gaithersburg, MD) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 0.1 mg/ml streptomycin.
In Vitro Pull-Down Assay, Immunoprecipitation, and Western Blotting
Cells were plated at 60–70% confluency and transfected using Effectene reagent (QIAGEN, Valencia, CA) following the manufacturer’s protocol. For IPs, cells were lysed in immunoprecipitation buffer [0.5% Triton X-100, 20 mm HEPES (pH 7.4), 150 mm NaCl, 2 mm dithiothreitol (DTT), 1 mm phenylmethylsulfonyl fluoride (PMSF)] containing a protease inhibitor cocktail (Roche Diagnostics GmbH, Mannheim, Germany). A total of 500 μg of whole cell lysate was immunoprecipitated using protein A/G agarose (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and Flag-specific M2 agarose (Sigma). Western analyses were performed using commercial antibodies including anti-HDAC3 monoclonal antibody (BD Transduction Laboratories, San Jose, CA), HDAC3 polyclonal antibody, and Flag-specific monoclonal and polyclonal antibodies (Sigma). All in vitro translations and labeling with [35S]methionine were performed using the indicated expression constructs and the TNT T7 quick-coupled transcription/translation kit from Promega Corp. (San Luis Obispo, CA), but a TNT Sp6 quick-coupled transcription/translation kit was used for TBL3 expression. GST pull-down protocols for analysis of protein-protein interaction were descried previously (7), and a GST fusion protein and in vitro-translated [35S]methionine-labeled proteins were used in this study. Biotinylated histone peptides were purchased from Upstate Biotechnology, Inc. (Charlottesville, VA). In the biotinylated histone peptide-binding assay, 1 μg of biotinylated H3, AcH3, H4, or AcH4 tail peptide was bound to streptavidin-agarose beads (Invitrogen, Carlsbad, CA), and pull-down assays were performed with in vitro-translated proteins. The binding assays were preformed at 4 C for 2 h in binding buffer [20 mm Tris-HCl (pH 7.1), 120 mm KCl, 1 mm DTT, 1 mm EDTA, 0.1% Nonidet P-40 (NP-40), 10% glycerol, 1 mm PMSF].
ChIP Assays
First, we isolated chromatin as described previously (28). Approximately 2 × 109 293T cells in 150-mm dishes were first treated with PBS containing 1% formaldehyde for 10 min, washed twice with cold PBS, and then incubated with 100 mm Tris (pH 9.4) and 10 mm DTT at 30 C for 15 min. The cells were then rinsed twice in PBS and resuspended in 600 μl of solution A [10 mm HEPES (pH 7.9), 0.5% NP-40, 1.5 mm MgCl2, 10 mm KCl, 0.5 mm DTT] by pipetting. After a short spin, the pellets were resuspended in solution B [20 mm HEPES (pH 7.9), 25% glycerol, 0.5% NP-40, 0.42 m NaCl, 1.5 mm MgCl2, 0.2 mm EDTA] containing protease inhibitors followed by vigorous pipetting to extract nuclear proteins. After centrifugation at 13,000 rpm for 30 min, the nuclear pellets were resuspended in immunoprecipitation buffer [1% Triton X-100, 2 mm EDTA, 20 mm Tris-HCl (pH 8.0), 150 mm NaCl, and protease inhibitors] and sonicated to break chromatin into fragments of 0.5–1 kb average length. The ChIP assays were then preformed with the indicated antibodies essentially as described, but without sodium dodecyl sulfate in all buffers.
Protein Cross-Linking Assays
Equal amounts of in vitro-translated proteins were immunoprecipitated with Flag-M2 beads and incubated in a buffer containing 20 mm HEPES (pH 7.6), 1.5 mm MgCl2, 50 mm NaCl, 10% glycerol, 0.5 mm PMSF, 0.2 mm EDTA, 20 mm DTT, and 0.05% NP-40 with various concentrations of glutaraldehyde (Sigma) at 37 C for 20 min. Cross-linking reactions were stopped by adding SDS-PAGE sample buffer and analyzed by SDS-PAGE and autoradiography.
NURSA Molecule Pages:
Coregulators: HDAC3 | NCOR | TBL1 | TBLR1;
Ligands: Thyroid hormone;
Nuclear Receptors: TRα.
Footnotes
This work was supported by a Seoul Science Fellowship (to H.G.C.) and by a grant (R13-2002-054-04002-0) from the Basic Research Program of the Korea Science and Engineering; the Korea Science and Engineering Foundation (KOSEF) grant by the Korea Government (MOST) (KOSEF 2007-8-1158); and Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2006-E00036) (to H.G.Y.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 17, 2008
Abbreviations: ChIP, Chromatin immunoprecipitation; D1, deiodinase 1; DTT, dithiothreitol; GST, glutathione-S-transferase; HDAC, histone deacetylase; LisH, Lis homology; N-CoR, nuclear receptor corepressor; NP-40, Nonidet P-40; PMSF, phenylmethylsulfonylfluoride; SMRT, silencing mediator for retinoic and thyroid receptor; TBL1, transducin β-like protein 1; TBLR, transducin β-like protein 1 receptor; TR, thyroid hormone receptor.
References
- 1.Chen JD, Evans RM 1995. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 337:454–457 [DOI] [PubMed] [Google Scholar]
- 2.Horlein AJ, Naar AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Soderstrom M, Glass CK 1995. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 377:394–404 [DOI] [PubMed] [Google Scholar]
- 3.Glass CK, Rosenfeld MG 2000. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev 14:121–141 [PubMed] [Google Scholar]
- 4.Li J, Wang J, Wang J, Nawaz Z, Liu JM, Qin J, Wong J 2000. Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC. EMBO J 19:4342–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Lin Q, Wang W, Wade P, Wong J 2002. Specific targeting and constitutive association of histone deacetylase complexes during transcriptional repression. Genes Dev 15:687–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishizuka T, Lazar MA 2003. The N-CoR/histone deacetylase 3 complex is required for repression by thyroid hormone receptor. Mol Cell Biol 23:5122–5131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon HG., Chan DW, Huang ZQ, Li J, Fondell JD, Qin J, Wong J 2003. Purification and functional characterization of the human N-CoR complex: the roles of HDAC3, TBL1 and TBLR1. EMBO J 17:1336–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Kalkurn M, Chait BT, Roeder RG 2002. The N-CoR-HDAC3 nuclear receptor corepressor complex inhibits the JNK pathway through the integral subunit GPS2. Mol Cell 9:611–623 [DOI] [PubMed] [Google Scholar]
- 9.Guenther MG, Lane WS, Fischle W, Verdin E, Lazar MA, Shiekhattar R 2000. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev 14:1048–1057 [PMC free article] [PubMed] [Google Scholar]
- 10.Bassi MT, Ramesar RS, Caciotti B, Winship IM, De Grandi A, Riboni M, Townes PL, Beighton P, Ballabio A, Borsani G 1999. X-linked late-onset sensorineural deafness caused by a deletion involving OA1 and a novel gene containing WD-40 repeat. Am J Hum Genet 64:1604–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan HT, Shinka T, Kinoshita K, Sato Y, Umeno M, Chen G, Tsuji K, Unemi Y, Yang XJ, Iwamoto T, Nakahori Y 2005. Molecular analysis of TBL1Y, a Y-linked homologue of TBL1X related with X-linked late-onset sensorineural deafness. Hum Mol Genet 50:175–181 [DOI] [PubMed] [Google Scholar]
- 12.Boulton SJ, Brook A, Staehling-Hampton K, Heitzlar P, Dyson N 2000A role for Ebi in neuronal cell cycle control. EMBO J 16:5376–5386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pijnappel WW, Schaft D, Roguev A, Shevchenko A, Tekotte H, Wilm M, Rigaut G, Seraphin B, Aasland R, Steward AF 2001. The S. cerevisiae SET3 complex includes two histone deacetylases, Hos2 and Hst1, and is a meiotic-specific repressor of the sporulation gene program. Genes Dev 15:2991–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perissi V, Aggarwal A, Glass CK, Rose DW, Rosenfeld MG 2004. A corepressor/coactivator exchange complex required for transcriptional activation by nuclear receptors and other regulated transcription factors. Cell 20:511–526 [DOI] [PubMed] [Google Scholar]
- 15.Cerna D, Wilson DK 2005. The structure of Sif2p, a WD repeat protein functioning in the SET3 corepressor complex. J Mol Biol 26:923–935 [DOI] [PubMed] [Google Scholar]
- 16.Chen G, Nguyen PH, Courey AJ 1998. A role for Groucho tetramerization in transcriptional repression. Mol Cell Biol 18:7259–7268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song H, Hasson P, Paroush Z, Courey AJ 2004. Groucho oligomerization is required for represson in vivo. Mol Cell Biol 24:4341–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emes RD, Ponting CP 2001. A new sequence motif linking lissencephaly, Treacher Collins and oral-facial-digital type 1 syndromes, microtubule dynamics and cell migration. Hum Mol Genet 10:2813–2820 [DOI] [PubMed] [Google Scholar]
- 19.Kim MH, Cooper DR, Oleksy A, Devedjiev Y, Derewenda U, Reiner O, Otlewski J, Derewenda ZS 2004. The structure of the N-terminal domain of the product of the lissencephaly gene Lis1 and its functional implications. Structure 12:987–998 [DOI] [PubMed] [Google Scholar]
- 20.Gerlitz G, Darhin E, Giorgio G, Franco B, Reiner O 2005. Novel functional features of the Lis-H domain: role in protein dimerization, half-life and cellular localization. Cell Cycle 4:1632–1640 [DOI] [PubMed] [Google Scholar]
- 21.Yoon HG, Choi Y, Cole PA, Wong J 2005. Reading and function of a histone code involved in target corepressor complexes for repression. Mol Cell Biol 25:324–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei Y, Jin J, Harper JW 2003. The cyclin E/Cdk2 substrate and Cajal body component p220(NPAT) activates histone transcription through a novel LisH-like domain. Mol Cell Biol 23:3669–3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edmodson DG, Smith MM, Roth SY 1996. Repression domain of the yeast global repressor Tup1 interacts directly with histones H3 and H4. Genes Dev 15:1247–1259 [DOI] [PubMed] [Google Scholar]
- 24.Wu J, Suka N, Carlson M, Grunstein M 2001. TUP1 utilizes histone H3/H2B-specific HDA1 deacetylase to repress gene activity in yeast. Mol Cell 7:117–126 [DOI] [PubMed] [Google Scholar]
- 25.Jabet C, Sprague ER, VanDemark AP, Wolberger C 2000. Chracterization of the N-terminal domain of the yeast transcriptional repressor Tup1. Proposal for an association model of the repressor complex Tup1X Ssn6. J Biol Chem 275:9011–9018 [DOI] [PubMed] [Google Scholar]
- 26.Edmondson DG, Zhang W, Watson A, Xu W, Bone JR, Yu Y, Stillman D, Roth SY 1998. In vivo functions of histone acetylation/deacetylation in Tup1p repression and Gcn5p activation. Cold Spring Harb Symp Quant Biol 63:459–468 [DOI] [PubMed] [Google Scholar]
- 27.Yoon HG, Chan DW, Reynolds AB, Qin J, Wong J 2003. N-CoR mediates DNA methylation-dependent repression through a methyl CpG binding protein Kaiso. Mol Cell 12:723–734 [DOI] [PubMed] [Google Scholar]
- 28.Zhang Q, Vo N, Goodman RH 2000. Histone binding protein RbAp48 interacts with a complex of CREB binding protein and phosphorylated CREB. Mol Cell Biol 20:4970–4978 [DOI] [PMC free article] [PubMed] [Google Scholar]