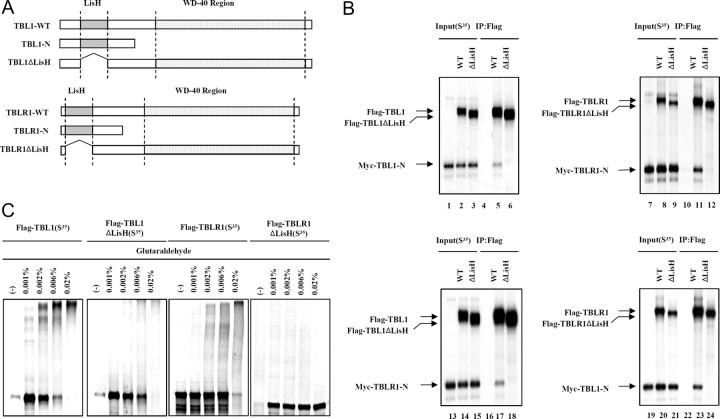
Fig. 1.
An N-Terminal LisH Domain in TBL1/TBLR1 Is Required for Oligomerization
A, Schematic of various full-length or truncated [35S]methionine-labeled TBL1 and TBLR1 proteins that were produced by in vitro translation. B, In vitro coimmunomobilization assays. A Myc-tagged TBL1 N-terminal region containing LisH (Myc-TBL1-N) was in vitro cotranslated with Flag-tagged, wild-type, full-length TBL1 (WT) and TBL1 without LisH (TBL1ΔLisH) or translated alone (left upper panel). A Myc-tagged TBLR1 N-terminal region containing LisH (Myc-TBLR1-N) was in vitro cotranslated with Flag-tagged, wild-type, full-length TBLR1 (WT) and TBLR1ΔLis-H (TBLR1ΔLis-H) or translated alone (right upper panel). The Myc-tagged TBLR1 N-terminal region containing the LisH domain (Myc-TBLR1-N) was in vitro cotranslated with Flag-tagged, wild-type, full-length TBL1 (WT) and TBL1ΔLisH or translated alone (left lower panel). The Myc-tagged TBL1 N-terminal region containing the LisH domain (Myc-TBL1-N) was in vitro cotranslated with Flag-tagged, wild-type, full-length TBLR1 (WT) and TBLR1ΔLisH or translated alone (right lower panel). The translation products were incubated with Flag M2-agarose beads. After extensive washing, Flag-bound proteins were eluted and analyzed by SDS-PAGE and autoradiography. C, Equal amounts of [35S]methionine-labeled proteins plus DTT were incubated with various concentrations of glutaraldehyde (indicated above each lane). Cross-linked products were resolved by SDS-PAGE and visualized by Coomassie blue staining and analyzed by autoradiography. IP, Immunoprecipitation.