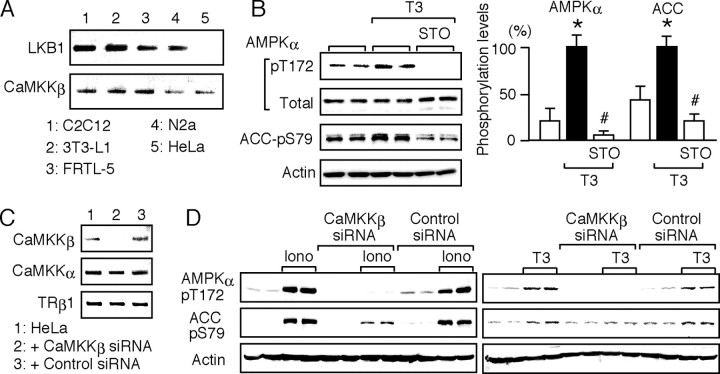
Fig. 3.
CaMKKβ Mediates the T3-Induced Phosphorylation of AMPK
A, The mRNA levels of LKB1 and CaMKKβ in C2C12, 3T3-L1, FRTL-5, N2a, and HeLa cells were determined by RT-PCR. The products were subjected to agarose gel electrophoresis and visualized by ethidium bromide. No band was detected when the reverse transcriptase was omitted (data not shown). B, HeLa cells were cultured without serum for 12 h. They were pretreated for 15 min with 1 μm STO-609 and treated for 30 min with 10 nm T3 in the presence of STO-609. Whole-cell lysates were subjected to Western blot analysis using antibodies against phospho-AMPK α-subunit (pT172), AMPK α-subunit (Total), phospho-ACC (pS79), and actin. Experiments were performed in duplicate cultures. Similar results were obtained from separate experiments. In densitometric analysis, the phospho-AMPK and phospho-ACC levels were normalized to the actin levels and expressed as percentages of the levels in cells treated with T3 alone. Results are means ± sd (n = 4). *, P < 0.05 vs. the control levels; #, P < 0.05 vs. the levels in cells treated with T3 alone. C, CaMKKβ siRNA and nontargeting, negative control siRNA were transfected into HeLa cells. The expression of CaMKKβ, CaMKKα, and TRβ1 was determined by RT-PCR 48 h after transfection. D, HeLa cells transfected with CaMKKβ or control siRNA for 48 h were cultured without serum for 12 h and then treated with 1 μm ionomycin or 10 nm T3 for 30 min. Whole-cell lysates were subjected to Western blot analysis using antibodies against phospho-AMPK α-subunit (pT172), phospho-ACC (pS79), and actin. The experiments were performed in duplicate cultures. Similar results were obtained from separate experiments.