Abstract
Steroids are synthesized mainly from the adrenal glands catalyzed by steroidogenic enzymes; the expression of these enzymes is controlled by transcription factor steroidogenic factor-1 (SF-1; NR5A1). To understand the physiological effect of genetic changes on steroid secretion, we used Cre-LoxP and gene targeting technology to mutate the binding sequence for SF-1 (SF-1 response element) on the promoter of the mouse Cyp11a1 gene, which encodes a critical enzyme for steroid biosynthesis. The resulting Cyp11a1L/L mice expressed about 7-fold less cytochrome P450 side-chain cleavage enzyme (CYP11A1) in the adrenal and testis but expressed normal amounts of CYP11A1 in the placenta and ovary. This tissue-specific reduction of gene expression did not affect basal steroid secretion but attenuated the circadian rhythm of glucocorticoid secretion. These mice also failed to induce glucocorticoid secretion in response to stress, leading to retention of CD4+CD8+ double-positive thymocytes. Unlike complete Cyp11a1 disruption, which causes neonatal death, promoter mutation did not decrease life span and caused no defect in reproduction. Thus, CYP11A1 appears in normal mice to be expressed above the minimal required level, providing a large capacity for use in response to stress. Mutation of the SF-1 response element of Cyp11a1 results in reduced stress response due to decreased adrenal CYP11A1 expression and insufficient stress-induced glucocorticoids secretion.
STEROIDS ARE SYNTHESIZED mainly in the adrenal cortex by the steroidogenic pathway. In this pathway, steroids are converted from a common precursor, cholesterol, by the action of various steroidogenic enzymes of which cytochrome P450 cholesterol side-chain cleavage enzyme (CYP11A1, also known as P450scc) is the first enzyme (1). Human patients with CYP11A1 mutations suffer from adrenal failure (2, 3), whereas Cyp11a1-null mice die shortly after birth, indicating that steroids are vital to maintain life (4).
Cyp11a1 is expressed abundantly in adrenals, gonads, placenta, and the central nervous system (5, 6). The adrenal- and gonad-specific expression of Cyp11a1 is controlled by transcription factor steroidogenic factor-1 (SF-1, now termed NR5A1). Two functional SF-1 binding sites [SF-1 response element (SF1RE)], P (proximal) and U (upstream), located at −40 and −1600 regions of human CYP11A1 promoter, are important for gene expression (7, 8). The P site is essential for basal transcription, and both P and U sites participate in hormone-responsive gene transcription in the adrenal and testis (8).
The same P site is also important for Cyp11a1 expression in placental giant trophoblast cell culture, although it binds a different transcription factor, activator protein-2 (AP-2) (5). In the ovary, it binds another ovarian protein, liver receptor homolog-1 (LRH-1), which has been considered to be the probable steroidogenic regulator activating Cyp11a1 expression (9).
Although the function of SF1RE has been analyzed in vitro and in transgenic mice (7, 8), its physiological function in vivo has never been investigated. In an effort to understand the effects of genetic changes on steroid secretion, in this report, we mutated the P site of Cyp11a1 promoter by gene targeting. We obtained surprising results showing reduced Cyp11a1 expression only in the adrenal and testis of mutant mice but not in the ovary and placenta. Although mutant mice were normal in their fertility, decreased Cyp11a1 expression led to attenuated adrenal circadian rhythms and reduced stress responses.
RESULTS
Mutation of the Proximal SF-1 Binding Site in the Mouse Cyp11a1 Promoter
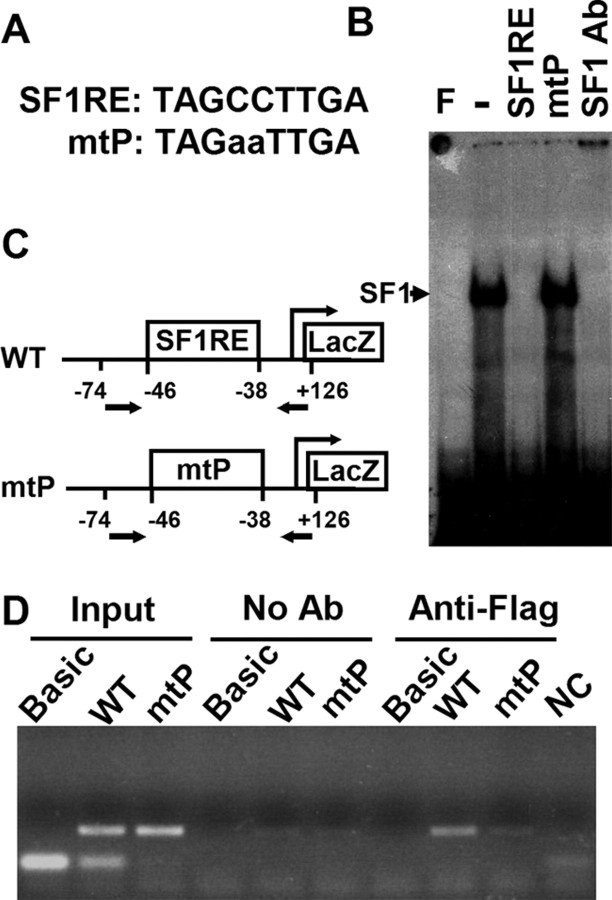
To understand the physiological function of SF1RE, we mutated two nucleotides in the proximal SF1RE at −38 to −46 of the mouse Cyp11a1 promoter (Fig. 1A) and examined the mutant’s ability to bind protein by gel shift analysis. Radiolabeled mouse SF1RE formed a complex with proteins in the Y1 nuclei extract (Fig. 1B). SF-1 antibody blocked the formation of this DNA-protein complex, demonstrating the presence of SF-1 in the complex. In addition, the formation of this complex was competed away by unlabeled mouse SF1RE but not by unlabeled mutant oligo (mtP). Thus, this mtP mutation prevents the binding of SF-1 to DNA in vitro.
Fig. 1.
Mutation of the Proximal SF-1 Binding Site Destroyed Its Ability to Bind SF-1
A, Sequences of SF-1 binding site (SF1RE) in the mouse Cyp11a1 proximal promoter. The mutated sequence in mtP is represented by lowercase letters. B, Gel shift analysis for mtP binding ability. Y1 nuclear extract was incubated with 32P-labeled SF1RE, and the protein-DNA complex was competed with unlabeled SF1RE or mtP or incubated with antibody against SF-1 (SF-1 Ab). F, Free probe without protein extract. C and D, Recruitment of endogenous SF-1 to the promoter. Wild-type (WT) or mtP mouse Cyp11a1 2-kb promoter linked to the LacZ vector or the LacZ vector alone without the promoter (Basic) was transfected into stable Y1 cell clones expressing 3xFLAG-SF-1. DNA was immunoprecipitated with antibodies against FLAG before analysis by PCR using primers that cover the regions between −74 of the Cyp11a1 promoter to +126 of LacZ. C, Diagram of the Cyp11a1 promoter region. Arrows indicate the locations of the primers used for PCR analysis. D, Gel analysis of the PCR products showing recruitment of Flag-SF-1 to the wild-type but not mtP promoter. Input represents control PCR of DNA samples before immunoprecipitation. No Ab represents control immunoprecipitation in the absence of antibodies. NC, Negative control for PCR without the addition of any DNA.
The ability of the mutant promoter to bind to SF-1 in vivo was further analyzed. Wild-type or mutant promoter linked to a Basic LacZ vector was transfected into a Y1 cell clone that overexpresses 3x-Flag-SF-1 (10). We used this cell line because its endogenous Flag-SF-1 can be easily immunoprecipitated by the anti-Flag antibody, thus alleviating the need for a good quality antibody against SF-1 suitable for immunoprecipitation, which is not currently available. The amount of endogenous Flag-SF-1 bound to the wild-type or mutated DNA was assessed after immunoprecipitation with the Flag antibody followed by analysis of DNA by PCR (Fig. 1C). The input control examined the efficiency of PCR before immunoprecipitation and showed that the primers spanning SF1RE amplified DNA from both the wild-type and mutant mtP promoters but not from control Basic vector, which does not contain the promoter sequence. When immunoprecipitation was performed in the absence of antibodies, no DNA was precipitated. In the presence of the Flag antibody, wild-type promoter was coprecipitated, whereas mtP promoter was not. This experiment showed that in vivo mutant promoter mtP failed to recruit SF-1.
Generation of Mice with Proximal SF1RE Mutation
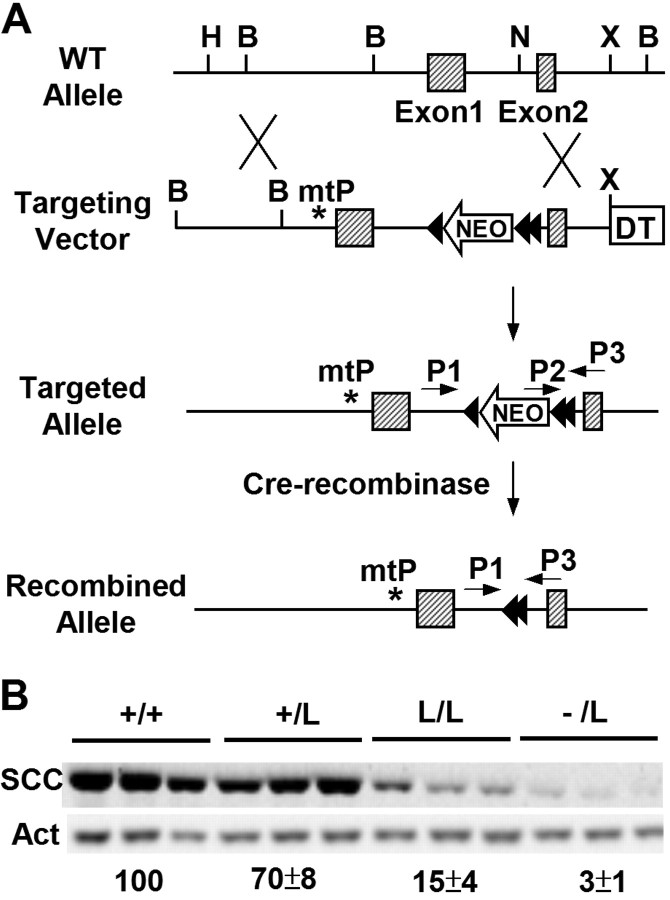
The mutation at SF1RE of Cyp11a1 was introduced into mice through homologous recombination in embryonic stem cells. During this process, the targeting vector contains a neo gene inserted into intron 1 of Cyp11a1 as a positive selection marker (Fig. 2A). The resulting cells were then used to generate knock-in mice with a mutation in the proximal SF1RE mutation. Subsequently, the neo insertion in the mice was excised by the Cre recombinase. The final mouse line has a mutation in the proximal SF-1 binding site and the insertion of one and a half loxP sites in the intron 1 of Cyp11a1. This allele was named mtP-L (abbreviated as L).
Fig. 2.
Generation of Mice with Mutations in Cyp11a1 Promoter Using the Cre-LoxP Strategy
A, Targeting strategy. The targeting vector contained a mutation in the proximal SF-1 binding site (mtP), an insertion of the neo gene flanked with loxP (arrowhead) sites in intron 1, and the diphtheria toxin (DT) gene at the 3′ end for negative selection. The neo insertion in targeted alleles was later removed by Cre recombinase. B, BamHI; H, HindIII; N, NcoI; X, XbaI. B, CYP11A1 (SCC) and β-actin (Act) expression in the adrenal glands. Mouse genotypes are shown above; each lane represents adrenal extract from one mouse. The relative CYP11A1 expression levels in each genotype after normalization to β-actin levels are shown below.
We have previously generated Cyp11a1−/− mice (4). By intercrossing Cyp11a1+/L mice or Cyp11a1L/L mice with Cyp11a1+/− mice, we generated two mouse lines, Cyp11a1L/L and Cyp11a1−/L. The adrenal expression levels of CYP11A1 in these mouse lines were determined by Western blotting followed by densitometric quantitation (Fig. 2B). Compared with their wild-type littermates, adrenal expression of CYP11A1 was reduced to 15% in Cyp11a1L/L mice and 3% in Cyp11a1−/L mice. Thus, we have generated mice that express less CYP11A1 in the adrenal glands through the creation of a mutation in the SF-1 binding site.
The expression levels of CYP11A1 in the gonads were also examined. Similar to that in the adrenal, expression of CYP11A1 in the testis was reduced in Cyp11a1L/L mice when compared with their wild-type littermates (Fig. 3A). The CYP11A1 expression levels in the ovary and placenta, however, were not affected by the mutation (Fig. 3A). Thus, the mutation of SF1RE in the Cyp11a1 promoter appears to have different effects in different tissues.
Fig. 3.
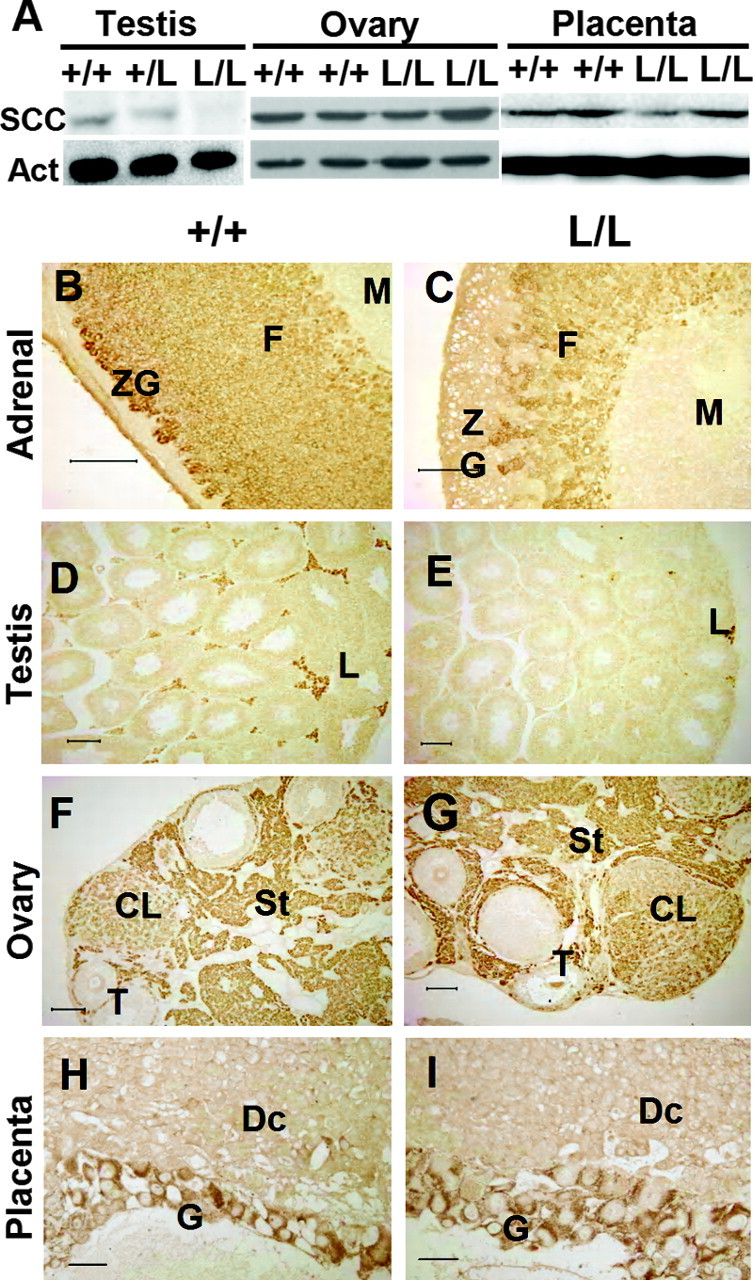
Tissue-Specific Reduction of CYP11A1 Expression in Cyp11a1L/L Mice
A, Western blot analysis of CYP11A1 (SCC) expression levels in ovary, placenta, and testis. Actin is used as an internal control. B–I, Immunohistochemistry of CYP11A1 expression, which is reduced in mutant (L/L) adrenal gland (C) and testis (E) but normal in the ovary (G) and placenta (I) compared with their wild-type counterparts (B, D, F, and H). Scale bar, 100 μm. CL, Corpus luteum; Dc, decidua; F, zona fasciculata; G, giant cells; L, Leydig cells; M, medulla; St, stroma; T, theca; ZG, zona glomerulosa.
Immunohistochemical staining was employed to further characterize the effect of promoter mutation on Cyp11a1 expression. CYP11A1 was detected in both zonae glomerulosa and fasciculata of the adult wild-type adrenal gland (Fig. 3B). In Cyp11a1L/L mice, this expression was reduced in both adrenal zones; the zona glomerulosa seemed to be more severely affected (Fig. 3C). In the testis, CYP11A1 signal was found in the Leydig cells (Fig. 3D), but it was reduced in a high proportion of Cyp11a1L/L Leydig cells (Fig. 3E). In the Cyp11a1L/L ovary, CYP11A1 expression in the corpus luteum, theca, and stroma was as strong as in the wild-type ovary (Fig. 3, F and G). CYP11A1 expression in the giant trophoblast cells of wild-type and Cyp11a1L/L placenta was also equally strong (Fig. 3, H and I). These results confirm our Western blot data showing that mutation of the proximal SF-1 binding site in the promoter resulted in reduced CYP11A1 expression in the adrenal and testis but had no effect in the ovary and placenta.
Because the expression of CYP11A1 was reduced in the adrenal of Cyp11a1L/L mice, we tested whether this caused a reduction of adrenal CYP11A1 enzymatic activity. The adrenals from Cyp11a1+/+ and Cyp11a1L/L mice were cultured in the presence of 20α-hydroxycholesterol as a substrate, and the formation of pregnenolone by the catalysis of CYP11A1 was measured. The Cyp11a1L/L adrenal produced lower levels of pregnenolone than the wild-type adrenal (Table 1), indicating reduced adrenal enzymatic activity. Thus, consistent with the reduction in protein amounts, the enzymatic activity of the Cyp11a1L/L adrenal was also reduced.
Table 1.
Cyp11a1L/L Mice Have Lower Adrenal CYP11A1 Enzymatic Activity but Normal Circulating Steroid Hormones Levels
| Hormones | Cyp11a1 +/+ | Cyp11a1 L/L |
|---|---|---|
| CYP11A1 enzymatic activity | ||
| Pregnenolone (ng/adrenal) | 7.1 ± 2.2 (n = 4) | 2.6 ± 0.4 (n = 4)1 |
| Steroid hormones | ||
| Pregnenolone (ng/ml) | 3.9 ± 0.5 (n = 8) | 5.9 ± 1.0 (n = 7) |
| Progesterone (ng/ml) | 73 ± 19 (n = 7) | 40 ± 6 (n = 7) |
| Aldosterone (ng/ml) | 185 ± 28 (n = 11) | 148 ± 19 (n = 6) |
| Corticosterone (ng/ml) | 18.4 ± 2.6 (n = 8) | 17.1 ± 0.9 (n = 8) |
Adrenal CYP11A1 enzymatic activity assay was performed by incubating adrenals with 20α-hydroxycholesterol, and the secretion of pregnenolone into the culture medium by the catalysis of CYP11A1 was measured. To measure blood steroid hormone levels, blood was withdrawn from mice at 0900 h and steroids were measured by commercial RIA or EIA kits. All values are shown as the mean ± sem.
P < 0.01.
Cyp11a1L/L Mice Have Normal Life Span and Normal Fertility
The Cyp11a1L/L mice had similar life span to the wild-type mice, but about 40% of these mutant mice died readily before the age of 5 wk. This fragility seems to be associated with the status of nursing. Cyp11a1L/L mice could survive to adulthood and lived as long as their wild-type littermates if they were given enough nursing by their mother when their litter size was reduced to around half. This indicates that Cyp11a1L/L mice have a normal life span if they pass through the initial difficulty. The survival of most Cyp11a1L/L mice indicates that lower than normal levels of CYP11A1 are not necessarily lethal.
We further analyzed the fertility of Cyp11a1L/L mice by mating them with other mice. Both Cyp11a1L/L and Cyp11a1−/L mice in both genders delivered their offspring with similar litter size when compared with control mating pairs (Cyp11a1+/L × Cyp11a1+/L) (Table 2). Their frequency of pregnancy was also normal. Thus, the mtP mutation did not affect the fertility of mice.
Table 2.
Cyp11a1L/L Mice Maintain Normal Litter Size
| Mating Pairs (Female × Male) | Litter Size |
|---|---|
| +/L × +/+ | 6.1 ± 2.6 (n = 17) |
| +/L × +/L | 6.1 ± 1.5 (n = 28) |
| L/L × +/− | 5.7 ± 1.7 (n = 34) |
| +/− × L/L | 5.7 ± 2.5 (n = 33) |
| −/L × −/L | 5.7 ± 1.7 (n = 10) |
| +/+ × +/+ | 6.2 ± 0.21 |
Mice carrying the mtP mutant allele (L) in the Cyp11a1 gene were mated with other alleles in all combinations, and their litter sizes were counted. Values are shown as the mean ± sd.
Data are cited from Mouse Genome Informatics (MGI): http://www.informatics.jax.org/external/festing/mouse/docs/C57BL.shtml.
The normal fertility in female mice was expected because ovarian CYP11A1 expression was normal. It was, however, unexpected that male mice also had normal fertility. We examined sperm counts and sperm motility; none of these parameters had any apparent defect (data not shown). Testis histology also appeared normal even though Cyp11a1 expression in the Leydig cells was reduced (Fig. 3, D and E, and data not shown). Serum testosterone level in adult Cyp11a1L/L mice was also normal (4.2 ± 0.9 ng/ml, n = 6). The normal level of testosterone despite reduced Cyp11a1 expression can explain why these mutant mice have normal fertility.
Cyp11a1 Deficiency Blunts Corticosterone Circadian Rhythm and Stress Response
Because adrenal Cyp11a1 expression is reduced in the mutant mice, we checked whether this affected steroid concentration. Serum was withdrawn from both wild-type and mutant mice at 0900 h, and levels of adrenal steroids were measured. The concentrations of pregnenolone, progesterone, aldosterone, and corticosterone in the Cyp11a1L/L mice were not significantly different from those in the wild-type mice (Table 1). Thus, the reduced expression of Cyp11a1 does not appear to affect steroid output during daytime.
Mice are nocturnal animal with a circadian pattern of corticosterone secretion reaching the lowest level in the early morning followed by a slow increase to a maximum in the evening (Fig. 4A). Cyp11a1L/L mice, however, secreted only half the amounts of normal corticosterone at both 1400 and 1900 h, although a small circadian increase was noted. Therefore, insufficient adrenal CYP11A1 expression failed to support a nocturnal increase in corticosterone secretion.
Fig. 4.
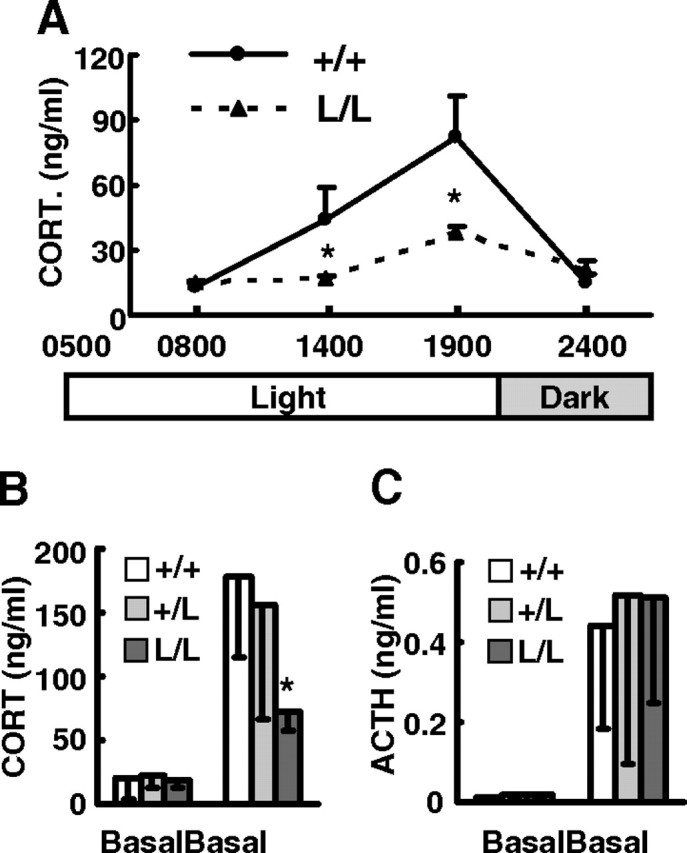
Cyp11a1L/L Mice Had Attenuated Circadian Corticosterone Rhythm and Stress Response
A, Plasma corticosterone concentrations from 8-wk-old +/+ (n = 4 or 5) and L/L (n = 6 or 7) male mice at different times of the day; B and C, plasma corticosterone concentration (B) and plasma ACTH concentration (C) in 8- to 12-wk-old +/+ (n = 8), +/L (n = 8 or 10), and L/L (n = 8) male mice at basal conditions or restrained in 50-ml conical tubes for 10 min are shown as mean ± sem. *, P < 0.05; **, P < 0.01 compared with the wild-type mice.
Corticosterone is a stress hormone, which is secreted upon stimulation by a stress (11). To assess the response of corticosterone secretion to stress, mice were restrained for 10 min to create an acute mild stress, and their plasma corticosterone levels were examined. Although the basal corticosterone levels were comparably low and were stimulated by restraint stress in wild-type and Cyp11a1+/L mice, the stimulated corticosterone levels in Cyp11a1L/L mice were less than half the amounts of controls (Fig. 4B). Plasma ACTH levels, on the contrary, were equally increased in all mice after restraining (Fig. 4C). This indicates that all these mice sense and respond to stress in the pituitary by secreting ACTH, but Cyp11a1L/L mice fail to secrete enough corticosterone upon ACTH stimulation because of the decreased CYP11A1 level in their adrenals.
Abnormal Cyp11a1L/L T Lymphocyte Development When Stressed
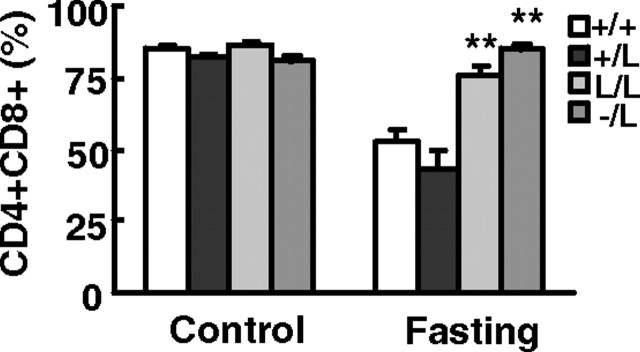
Glucocorticoids induce the apoptosis of CD4+CD8+ double-positive thymocytes during thymocyte development (12). We analyzed the composition of developing T cell precursors in mouse thymus after 48-h starvation stress. Fasting caused a decrease in double-positive thymocytes in wild-type mice, but the percentage of CD4+CD8+ thymocytes in Cyp11a1L/L and Cyp11a1−/L mice either did not decrease much or was unaffected (Fig. 5). Therefore, the apoptosis of CD4+CD8+ double-positive thymocytes is blocked when corticosterone secretion was impaired.
Fig. 5.
Altered thymocyte composition in stressed Cyp11a1L/L mice. Percentage of CD4+CD8+ cells in total thymocytes determined by FACS analysis in 9- to 12-wk-old +/+ (n = 8), +/L (n = 8), L/L (n = 8), and −/L (n = 7 or 9) male mice are shown as mean ± sem. *, P < 0.05; **, P < 0.01 when comparing mutants with wild-type (+/+) mice.
Low CYP11A1 Expression Perturbs Adrenal Structure
The adrenal glands are the organs responsible for circadian and stress-induced corticosterone secretion. We examined the histology of Cyp11a1L/L adrenal glands to see whether impaired stress response affects adrenal structure. The Cyp11a1L/L adult adrenal was slightly larger than the wild-type, and the cortical zonation was also slightly disturbed (Fig. 6, A and B). We also observed numerous vacuolar structures in zonae fasciculata and glomerulosa (Fig. 6B). There were large lipid droplets as revealed by Oil red O staining (Fig. 6, C and D). At the ultrastructural level, wild-type adrenocortical cells contained characteristic tubulo-vesicular internal membranes in the mitochondria. By contrast, in Cyp11a1L/L adrenocortical cells, this structure was poorly formed, and the cristae were not readily discernable (Fig. 6, E and F). Insufficient CYP11A1 expression thus results in disturbed adrenal structure, oil accumulation, and mitochondrial abnormality.
Fig. 6.
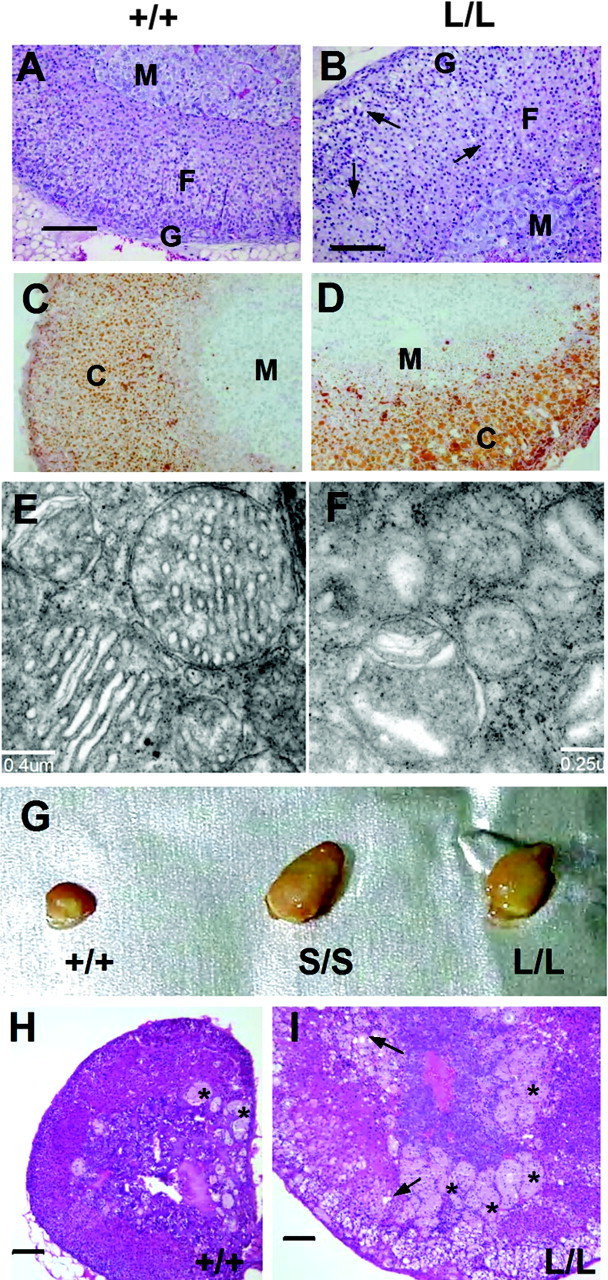
Adrenal Histology of Cyp11a1-Deficient Mice
A and B, Hematoxylin and eosin staining of 8-wk-old adrenal glands, with arrows indicating vacuoles; C and D, Oil-red-O staining; E and F, electron micrograms of adrenocortical mitochondria; G, adrenal glands of 18-month-old mice of various genotypes (indicated); H and I, adrenal histology of 18-month-old +/+ (H) and L/L (I) mice after hematoxylin and eosin staining. Arrows indicate vacuolar structures. Asterisks indicate syncytial lipoid structures. Scale bar, 100 μm (A, B, H, and I). C, Cortex; F, fasciculata; G, glomerulosa; M, medulla.
Old Cyp11a1L/L Mice Developed Adrenal Hyperplasia
We observed slightly larger adrenal glands in young Cyp11a1L/L mice and found that this condition worsened as mice became older. Figure 6G shows that the adrenal gland of an 18-month-old Cyp11a1L/L mouse was much bigger than the wild-type adrenal of the same age. Another mouse line, Cyp11a1S/S, which has the mutation in the SF1RE of the promoter with a shorter insertion of one LoxP sequence in the first intron, is also partially defective in corticosterone secretion (Hsu N.-C., M. C. Shih, C.-C. Huang, and B.-c. Chung, unpublished data); this Cyp11a1S/S mouse also had bigger adrenals. Histological analysis of these adrenal glands from older mice revealed severe hyperplasia and hypertrophy with the presence of many syncytial lipid structures at the junction between medulla and cortex (Fig. 6I). Although syncytial lipid structures were also found in wild-type adrenals, they were much smaller and less frequent. Thus, small deleterious disturbances to the adrenal glands were cumulative and became pathologically abnormal upon aging.
DISCUSSION
In this study, we created a mutation in the proximal SF-1-binding site of the Cyp11a1 promoter and found this mutation reduced adrenal and testicular gene expression but had no effect to the ovary and placenta. In addition, we found that reduced Cyp11a1 expression attenuated diurnal corticosterone secretion and reduced response to stress. This study provides a better understanding of the threshold requirement in different steroidogenic tissues for Cyp11a1 expression.
Differential Effects of Cyp11a1 SF1RE in Different Tissues
The mutant mice described in this report had a mutation in the proximal TAGCCTTGA sequence (P site), which binds transcription factors like SF-1 (13), LRH-1 (14), and AP-2 (5). This mutation resulted in reduced CYP11A1 expression in the adrenal and testis, confirming our earlier data obtained in cell culture (7) and in transgenic mice (8, 15). The inability of this mutation to affect ovarian and placental CYP11A1 expression, however, came as a surprise. This site was shown by deletion analysis to be important for rat Cyp11a1 expression in the ovarian cells (16, 17). In addition, it binds another ovarian protein, LRH-1, which has been considered to be the probable steroidogenic regulator in the ovary (9). Despite these data, our results unequivocally demonstrate that this P site alone is not required for CYP11A1 expression in the ovary. Although this P site is dispensable for Cyp11a1 expression, it may still have a role in transcription. Maybe many cis-regulatory sequences can stimulate gene expression simultaneously, and in the absence of this P site, other regulatory sequences can compensate for it in the ovary.
The control of CYP11A1 expression in the placenta is also not clearly understood. Placental AP-2 binds to the P site to activate CYP11A1 (5). Because our data show this P site is not important by itself, this cis-element is probably redundant. Many other placental factors, like the long terminal repeat binding protein (LBP-1b) and the 132-kDa transcriptional regulating protein (TReP-132) (18, 19), have been shown to play a role in CYP11A1 transcription via sites neighboring the P site. These are possible candidates that may take over the P-site binding protein to drive Cyp11a1 gene expression in placenta.
Physiological Requirement for Cyp11a1 Expression
Cyp11a1L/L mice grew normally and were fertile, although they expressed only 15% of the normal amounts of CYP11A1. This indicates that 15% of Cyp11a1 expression is sufficient to support life and that animals have evolved a large reservoir of CYP11A1 of which normally only a small amount is used to support basal activities. We show this large reservoir is required for stress response, under which condition larger amounts of CYP11A1 are needed for sufficient steroid secretion. This implies that up to 85% of CYP11A1 is kept in reserve for spontaneous reactions to stress.
We did not see any reproductive defects in male and female Cyp11a1L/L mice. This was expected of female Cyp11a1L/L mice, because Cyp11a1 expression in their ovary was normal. In the testis, despite reduced Cyp11a1 expression, testosterone secretion was still normal, and male Cyp11a1L/L mice reproduced normally. This indicates that the capacity of CYP11A1 in wild-type testis Leydig cells is very high, and a fraction of it is already adequate for supply of androgens. The lack of reproductive phenotype also indicates that contrary to our common belief, the proximal SF1RE in the Cyp11a1 promoter is dispensable for reproductive function.
Adrenal Structure Affected by Steroid Insufficiency
Cyp11a1L/L mice express low levels of CYP11A1 and exhibit a stress-related corticosterone insufficiency phenotype. This phenotype can also be found in SF-1 heterozygous mice (20). SF-1 heterozygous mice have smaller adrenals (21), yet Cyp11a1L/L mice have progressively larger adrenals. This is because the growth of Cyp11a1L/L adrenals is increased by raised ACTH, which may be stimulated from time to time by minor stresses in the animal room such as cage changes and occasional handling.
Our histological data showed both hyperplasia and hypertrophy. Hyperplasia is caused by long-term ACTH overstimulation. Hypertrophy is probably a pathological presentation of adrenal CYP11A1 deficiency. Indeed, we observed vacuoles in and around the adrenal cortex. These pathological changes were cumulative and worsened with the age of the animals.
Abnormal mitochondrial structure seems to be indicative of steroidogenic capacity of the cell. Although exhibiting tubulo-vesicular structure in wild-type steroidogenic cells, mitochondria in Cyp11a1L/L cells had reduced steroidogenic capacity and reduced number of cristae. The Cyp11a1−/− mitochondria without steroidogenic capability had completely lost cristae (22).
Consequences of Impaired Stress Response
Under prolonged stress, the number of CD4+CD8+ thymocytes is aberrantly higher in Cyp11a1L/L mice (Fig. 5). The elimination of double-positive thymocytes is an important step controlled by glucocorticoids during T cell development (23), and failure to do so could result in autoimmune diseases. Lewis rats with an impaired hypothalamic-pituitary-adrenal axis were more susceptible to streptococcal cell wall-induced arthritis (24). Thus, it will be interesting to see whether the Cyp11a1L/L mice have problems in immune functions such as susceptibility to autoimmune diseases.
MATERIALS AND METHODS
Gel Shift Assays
The gel shift experiments were performed using 10 μg Y1 cell extract for each reaction as previously described (7). Sequences of the oligos used in gel shift are as follows: SF1RE, CAGCTTCTCTCTTAGCCTTGAGCTGGTGG, and mtP, CAGCTTCTCTCTTAGAATTGAGCTGGTGG.
Plasmid
The Cyp11a1 promoter fragment (−2000 to ∼+8) was released from the 129/SvJ BAC clone by digestion with EcoRI and BssI. This fragment was first subcloned into pSK vector and then into pβgal-Basic vector after digestion with HindIII and SmaI. The mtP plasmid was generated from the pβgal-WT by primer-based mutagenesis. The mutant oligo is CCACCAGCTCAAttCTAAGAGAGAAGCTG.
In Vivo DNA Binding Assay
The recruitment of endogenous SF-1 protein to the promoter DNA was analyzed in a Y1 stable cell line that overexpresses 3xFLAG-SF-1 (10). These cells (7.5 × 105 cultured overnight in 100-mm plates) were transected with 3 μg wild-type or mutant plasmid and cultured for another 48 h. Cells were then cross-linked with 1% formaldehyde for 10 min before the reaction was stopped with 0.125 m glycine for 5 min at room temperature. Nuclei were collected by low-speed centrifugation, dissolved in 300 μl SDS buffer [50 mm Tris-HCl (pH 8.1), 10 mm EDTA, 1% SDS, 1× Complete protease inhibitor cocktail], and sonicated at high speed for 30 sec on and 30 sec off, six times, in Bioruptor (Diagenode Inc., Liège, Belgium). Soluble DNA was diluted 10× in dilution buffer [0.01% SDS, 1.1% Triton X-100, 1.2 mm EDTA, 16.7 mm Tris-HCl (pH 8.1), 167 mm NaCl, 1× Complete protease inhibitor cocktail] and precleared by incubation with 95 μl protein A agarose/salmon sperm DNA (50% slurry) (Millipore, Billerica, MA) for 2 h, and 200 μl of the solution was saved as input control. The rest of the samples were reacted with 1.5 μg anti-FLAG M2 antibody (Sigma Chemical Co., St. Louis, MO) followed by rabbit antimouse IgG overnight at 4 C and then incubated with 30 μl protein A agarose/salmon sperm DNA (50% slurry) for 1 h at 4 C. The agarose beads were washed two times with each of the following buffers for 4 min at 4 C: low-salt wash buffer [0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris-HCl (pH 8.1), 150 mm NaCl], high-salt wash buffer [0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris-HCl (pH 8.1), 500 mm NaCl], LiCl wash buffer [0.25 m LiCl, 1% NP-40, 1% deoxycholate, 1 mm EDTA, 10 mm Tris-HCl (pH 8.1)], and 1× Tris-EDTA buffer (pH 8). DNA was eluted from protein A agarose by 250 μl elution buffer (1% SDS, 0.1 m NaHCO3) two times at room temperature. The cross-link in DNA was reversed in 20 μl 5 m NaCl and 10 μg RNase A at 65 C for 4–6 h. DNA was then eluted in 10 μl 0.5 m EDTA, 20 μl 1 m Tris-HCl (pH 6.5), and 2 μl 10 mg/ml proteinase K at 55 C for 1 h. The precipitated DNA was cleaned up by phenol-chloroform extraction and 2-propanol precipitation and analyzed by 24 cycles of PCR amplification using primers spanning the proximal of mouse Cyp11a1 promoter and pβ-gal vector (forward, GAGGTCACCGCTCCATCAGC; reverse, CCAGACCAATGCCTCCCAGA).
Generation of Mice with Cyp11a1 Promoter Mutation
A 2-bp mutation changing the SF-1 binding site at −46/−38 of Cyp11a1 from TAGCCTTGA to TAGaaTTGA was generated by site-directed in vitro mutagenesis. This mutation was introduced into embryonic stem cells by homologous recombination followed by blastocyst injection. Heterozygous mice harboring the mutation were then selected and mated with EIIa-Cre mice, which contain universal Cre recombinase (25). The details for the generation of these mutant mice will be described elsewhere (Hsu, N.-C., M.-C. Shih, C.-C. Huang, and B.-c. Chung, manuscript in preparation). The resulting mutant mice, named Cyp11a1L/L, contain a mutation in the promoter and a concomitant insertion of one and a half loxP sites in the first intron. All the mice were backcrossed to wild-type C57BL/6 for four to 10 generations.
Animals and Treatments
Mice were housed in standard conditions in a 14-h light, 10-h dark cycle and were given food and water ad libitum following guidelines approved by the Institutional Animal Care and Use Committee. Lights were turned on at 0500 h and off at 1900 h. For restraint stress analysis, male mice were singly housed for 1 wk and then immobilized in 50-ml conical tubes for 10 min. For fasting experiments, 8- to 9-wk-old male mice were given water ad libitum but deprived of food for 48 h. For circadian corticosterone measurement, blood samples were collected from adult male mice within 2 min from the tail vein at the indicated times.
Enzyme Activity Assay
Adrenals were taken from male mice and cultured in 0.5 ml DMEM/F12 medium containing 10 μm Trilostane to block 3β-hydroxysteroid dehydrogenase activity. The substrate for CYP11A1, 20α-hydroxycholesterol (12.5 μm), was added into the medium 30 min later and incubated for another 4 h. CYP11A1 enzyme activity was determined by measuring the amounts of secreted pregnenolone from the medium by a commercial enzyme immunoassay (EIA) kit (ALPCO Diagnostics, Salem, NH).
Blood Analysis
All mice were singly housed for 1 wk before blood samples were collected either from tail veins or by decapitation within 1 min of disturbing the mice. Plasma ACTH, corticosterone, and TNFα were measured by RIA (for corticosterone from ICN Biomedicals, Inc., Palo Alto, CA; and for ACTH from Nichols Institute Diagnostics, San Juan Capistrano, CA) or ELISA (Endogen, Rockford, IL) kits according to the manufacturers’ instructions. Serum pregnenolone, progesterone, and testosterone were measured by EIA (for pregnenolone from ALPCO Diagnostics, and for progesterone from Assay Designs, Inc., Ann Arbor, MI) or RIA (for testosterone; DSL, Inc., Webster, TX) kits according to the manufacturers’ instructions.
Western Blotting
Mouse tissues were homogenized in lysis solution [100 mm potassium phosphate (pH 7.8) and 0.2% Triton X-100], and protein concentration was determined using the Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA). Proteins (10 μg) were separated by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked in 5% skim milk, reacted with rabbit antibody against human CYP11A1 (1:104) and then incubated with goat antirabbit horseradish peroxidase, followed by chemiluminescent detection.
Histological Analyses
Individual organs were fixed in Bouin’s solution for 24 h, embedded in tissue embedding medium Paraplast (TYCO Healthcare, Mansfield, MA), and sectioned. For morphological examination, tissue sections were stained with hematoxylin and eosin and Oil red O. For P450scc expression analysis, tissue sections were immunostained with antihuman CYP11A1 antibody (26) using the ABC kit (Vector Laboratories, Inc., Burlingame, CA), and visualized by staining with 3,3′-diaminobenzidine as previously described (5).
Flow Cytometry Analysis of Thymus Cell Subsets
Cells were released from individual thymus glands with a glass tissue grinder and prepared as single-cell preparations in RPMI medium containing 10% fetal calf serum. Thymus subsets were determined by staining single cells with fluorescein isothiocyanate-conjugated anti-CD4 (clone GK1.5; eBioscience, San Diego, CA) in combination with phycoerythrin-conjugated anti-CD8 (clone 53–6.7; eBioscience) monoclonal antibodies. The percentage of each cell subset was determined by flow cytometry using FACScalibur (BD Biosciences, San Diego, CA).
Ultrastructural Studies
Tissues were fixed in 2.5% glutaraldehyde in 0.1 m phosphate buffer (pH 7.2) overnight followed by washing in 0.1 m phosphate buffer (15 min, three times). After re-fixation in 1% osmium tetroxide for 2 h at room temperature, they were washed in phosphate buffer, dehydrated in a graded series (30, 50, 70, 80, 90, 95, and 100%) of acetone/phosphate buffer for 15 min each, equilibrated, and embedded in ERL 4206 epoxy resin. For transmission electron microscopic studies, ultrathin 80-nm sections were mounted on coated 50-mesh copper grids, contrast stained with aqueous solutions of uranyl acetate and lead citrate, and viewed and photographed using an FEI Tecnai TM G2 transmission electron microscope.
Statistical Analysis
All blood analysis data were evaluated by Student’s t test and are shown as mean ± sem. The cumulative survival rate was calculated using the Breslow Generalized Wilcoxon Test in SPSS software (SPSS Inc., Chicago, IL).
Acknowledgments
We thank Sin-Tak Chu for sperm count and motility test, Transgenic Core Facility at Academia Sinica for the generation of knockout mouse lines, and Yi-Lin Chien and Shu-Jan Chou for excellent technical assistance.
Footnotes
This work was supported by Academia Sinica, Taiwan (Grant AS92IMB4PP).
Current address for C.-C.H.: Department of Veterinary Biosciences, University of Illinois at Urbana-Champaign, Urbana, Illinois 61802.
Disclosure Statement: The authors have no financial or other conflicts of interest in the publication of this work.
First Published Online January 3, 2008
Abbreviations: CYP11A1, Cytochrome P450 cholesterol side-chain cleavage enzyme; EIA, enzyme immunoassay; LRH-1, liver receptor homolog-1; P, proximal; SF-1, steroidogenic factor-1; SF1RE, SF-1 response element; U, upstream.
References
- 1.Payne AH, Hales DB 2004. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev 25:947–970 [DOI] [PubMed] [Google Scholar]
- 2.Katsumata N, Ohtake M, Hojo T, Ogawa E, Hara T, Sato N, Tanaka T 2002. Compound heterozygous mutations in the cholesterol side-chain cleavage enzyme gene (CYP11A) cause congenital adrenal insufficiency in humans. J Clin Endocrinol Metab 87:3808–3813 [DOI] [PubMed] [Google Scholar]
- 3.Tajima T, Fujieda K, Kouda N, Nakae J, Miller WL 2001. Heterozygous mutation in the cholesterol side chain cleavage enzyme (p450scc) gene in a patient with 46,XY sex reversal and adrenal insufficiency. J Clin Endocrinol Metab 86:3820–3825 [DOI] [PubMed] [Google Scholar]
- 4.Hu MC, Hsu NC, El Hadj NB, Pai CI, Chu HP, Wang CK, Chung BC 2002. Steroid deficiency syndromes in mice with targeted disruption of Cyp11a1 Mol Endocrinol 16:1943–1950 [DOI] [PubMed] [Google Scholar]
- 5.Ben-Zimra M, Koler M, Orly J 2002. Transcription of cholesterol side-chain cleavage cytochrome P450 in the placenta: activating protein-2 assumes the role of steroidogenic factor-1 by binding to an overlapping promoter element. Mol Endocrinol 16:1864–1880 [DOI] [PubMed] [Google Scholar]
- 6.Furukawa A, Miyatake A, Ohnishi T, Ichikawa Y 1998. Steroidogenic acute regulatory protein (StAR) transcripts constitutively expressed in the adult rat central nervous system: colocalization of StAR, cytochrome P-450SCC (CYP XIA1), and 3β-hydroxysteroid dehydrogenase in the rat brain. J Neurochem 71:2231–2238 [DOI] [PubMed] [Google Scholar]
- 7.Guo IC, Tsai HM, Chung BC 1994. Actions of two different cAMP-responsive sequences and an enhancer of the human CYP11A1 (P450scc) gene in adrenal Y1 and placental JEG-3 cells. J Biol Chem 269:6362–6369 [PubMed] [Google Scholar]
- 8.Hu MC, Hsu NC, Pai CI, Wang CK, Chung B 2001. Functions of the upstream and proximal steroidogenic factor 1 (SF-1)-binding sites in the CYP11A1 promoter in basal transcription and hormonal response. Mol Endocrinol 15:812–818 [DOI] [PubMed] [Google Scholar]
- 9.Liu DL, Liu WZ, Li QL, Wang HM, Qian D, Treuter E, Zhu C 2003. Expression and functional analysis of liver receptor homologue 1 as a potential steroidogenic factor in rat ovary. Biol Reprod 69:508–517 [DOI] [PubMed] [Google Scholar]
- 10.Chen WY, Weng JH, Huang CC, Chung BC 2007. Histone deacetylase inhibitors reduce steroidogenesis through SCF-mediated ubiquitination and degradation of steroidogenic factor 1 (NR5A1). Mol Cell Biol 27:7284–7290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sapolsky RM, Romero LM, Munck AU 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89 [DOI] [PubMed] [Google Scholar]
- 12.Ashwell JD, Lu FW, Vacchio MS 2000. Glucocorticoids in T cell development and function. Annu Rev Immunol 18:309–345 [DOI] [PubMed] [Google Scholar]
- 13.Guo IC, Hu MC, Chung BC 2003. Transcriptional regulation of CYP11A1. J Biomed Sci 10:593–598 [DOI] [PubMed] [Google Scholar]
- 14.Kim JW, Havelock JC, Carr BR, Attia GR 2005. The orphan nuclear receptor, liver receptor homolog-1, regulates cholesterol side-chain cleavage cytochrome p450 enzyme in human granulosa cells. J Clin Endocrinol Metab 90:1678–1685 [DOI] [PubMed] [Google Scholar]
- 15.Guo IC, Huang CY, Wang CK, Chung BC 2007. Activating protein-1 cooperates with steroidogenic factor-1 to regulate 3′,5′-cyclic adenosine 5′-monophosphate-dependent human CYP11A1 transcription in vitro and in vivo. Endocrinology 148:1804-1812 [DOI] [PubMed] [Google Scholar]
- 16.Falender AE, Lanz R, Malenfant D, Belanger L, Richards JS 2003. Differential expression of steroidogenic factor-1 and FTF/LRH-1 in the rodent ovary. Endocrinology 144:3598–3610 [DOI] [PubMed] [Google Scholar]
- 17.Clemens JW, Lala DS, Parker KL, Richards JS 1994. Steroidogenic factor-1 binding and transcriptional activity of the cholesterol side-chain cleavage promoter in rat granulosa cells. Endocrinology 134:1499–1508 [DOI] [PubMed] [Google Scholar]
- 18.Gizard F, Lavallee B, DeWitte F, Hum DW 2001. A novel zinc finger protein TReP-132 interacts with CBP/p300 to regulate human CYP11A1 gene expression. J Biol Chem 276:33881–33892 [DOI] [PubMed] [Google Scholar]
- 19.Huang N, Miller WL 2005. LBP proteins modulate SF1-independent expression of P450scc in human placental JEG-3 cells. Mol Endocrinol 19:409–420 [DOI] [PubMed] [Google Scholar]
- 20.Bland ML, Jamieson CA, Akana SF, Bornstein SR, Eisenhofer G, Dallman MF, Ingraham HA 2000. Haploinsufficiency of steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response. Proc Natl Acad Sci USA 97:14488–14493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bland ML, Fowkes RC, Ingraham HA 2004. Differential requirement for steroidogenic factor-1 gene dosage in adrenal development versus endocrine function. Mol Endocrinol 18:941–952 [DOI] [PubMed] [Google Scholar]
- 22.Ishii T, Hasegawa T, Pai CI, Yvgi-Ohana N, Timberg R, Zhao L, Majdic G, Chung BC, Orly J, Parker KL 2002. The roles of circulating high-density lipoproteins and trophic hormones in the phenotype of knockout mice lacking the steroidogenic acute regulatory protein. Mol Endocrinol 16:2297–2309 [DOI] [PubMed] [Google Scholar]
- 23.Herold MJ McPherson KG, Reichardt HM 2006. Glucocorticoids in T cell apoptosis and function. Cell Mol Life Sci 63:60–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sternberg EM, Hill JM, Chrousos GP, Kamilaris T, Listwak SJ, Gold PW, Wilder RL 1989. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc Natl Acad Sci USA 86:2374–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H 1996. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci USA 93:5860–5865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu MC, Guo IC, Lin JH, Chung BC 1991. Regulated expression of cytochrome P-450scc (cholesterol-side-chain cleavage enzyme) in cultured cell lines detected by antibody against bacterially expressed human protein. Biochem J 813–817 [DOI] [PMC free article] [PubMed]