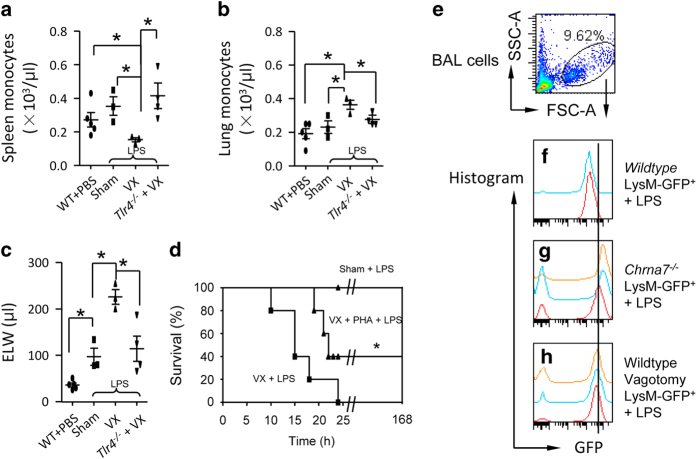
Figure 1.
Disruption of vagal circuits exacerbates LPS-induced ALI by enhancing egress of splenic monocytes and migration of granulocytes towards the injured lungs. (a–c) Effect of vagotomy on splenic and pulmonary monocytes, and extravascular lung water (ELW) in LPS-induced ALI. The mice were divided into four groups: WT (PBS IT), sham+LPS (5 mg kg−1 IT), vagotomy+LPS (5 mg kg−1 IT) or Tlr4−/−+vagotomy+LPS (5 mg kg−1 IT). Mice were killed at 15 h after intratracheal LPS to measure splenic monocytes (a), pulmonary monocytes (b) and ELW (c). N=3–5 in each group. Data were pooled from three experiments. *P<0.05, one-way ANOVA with Bonferroni post hoc test. Data are presented as mean±s.d. (d) Effect of vagotomy on mortality of LPS-induced ALI. The mice were divided into three groups: sham+LPS (n=8), vagotomized+LPS (n=9) and vagotomized+PHA568487+LPS (n=4). The mice were IT instilled with LPS at dose of 5 mg kg−1. Mice of the vagotomized+PHA568487+LPS group were given an intraperitoneal (ip) injection of PHA568487 (0.8 mg kg−1) 15 min before LPS, and repeated the same dose 6, 12 and 18 h, respectively, after LPS to maintain drug concentration in the blood. The corresponding vehicles were given in the other two groups. The mortality of mice was followed up for 7 days. *P<0.05 by log-rank test. (e–h) Effect of deficiency of α7 nAChR and vagotomy on LysM-GFP+ cell migration to the airspaces of the lung during LPS-induced ALI. The mice were divided as follows: wildtype LysM-GFP+ mice receiving an IT of LPS (5 mg kg−1), Chrna7−/−LysM-GFP+ mice receiving an IT of E. coli (5 mg kg−1), and vagotomized wildtype LysM-GFP+ mice receiving an IT of LPS (5 mg kg−1). The mice were killed at 24 h after LPS challenge. The BAL cells were collected for flow cytometry. The whole-cell population was gated (e). The LysM-GFP histogram was applied to each sample in wildtype LysM-GFP++LPS (n=2) (f), Chrna7−/−LysM-GFP++LPS (n=3) (g) and vagotomized LysM-GFP++LPS (n=3) (h).